Temperature - Warren County Schools
advertisement

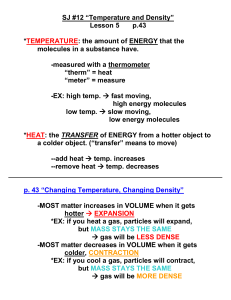
Not the same as heat!!! Heat Form of energy Quantity of energy within a system Heat flows from higher temp to lower temp Temperature Measure of intensity of heat SI unit for temp is Kelvin No degree symbol for Kelvin K K , °C, °F Mixture Retain individual properties May be physically separated Filtration Magnet Centrifuge solution Sift Distillation Fractional distillation Chromatography Electrophoresis size Evaporation solids from suspension magnetic suspended from large from small solvent from solution separate alcohol colors molecules based on solute from solution Element Can not be broken down Found on the periodic table Simplest form Building block of all substances Compound Combination of two or more elements Can not be separated by physical means Do not retain properties of their individual elements Molecule Smallest uncharged individual unit of a compound Formed by two or more atoms joined together Water All compounds are molecules but not all molecules are compounds Mixture Homogeneous Uniform Heterogeneous Two or more phases Properties Physical Color Taste Odor Physical existence State of matter Mp Density Bp Chemical Ability of substance to form a new substance Changes Physical Chemical Changes in physical New substance is formed properties Different set of properties Change in state of matter No new substance is formed Intensive Properties Do not depend on the amount of matter Color Odor Luster Malleability Ductility Conductivity Hardness Melting/Freezing Point Boiling Point Density Extensive Properties Depend on the amount of matter Mass Weight Volume Length