Chemistry of Art
advertisement

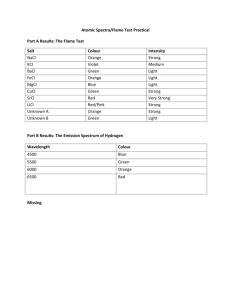
CHEMISTRY OF ART Light emission by Atoms COLOURS & FIRES • Stage 6 Chemistry Syllabus – Chemistry of Art (Option) • Identify Na+, K+, Ca2+, Ba2+, Sr2+, and Cu2+ by their flame colour • Perform first-hand investigations to observe the flame colour of Na+, K+, Ca2+, Ba2+, Sr2+, and Cu2+ (Demo only this lesson) • Explain the flame colour in terms of electrons releasing energy as they move to a lower energy level • Explain why excited atoms only emit certain frequencies of radiation • Explain what is meant by n, the principal quantum number • Identify that, as electrons return to lower energy levels, they emit quanta of energy which humans may detect as a specific colour WHAT’RE THOSE COLOURS? . • The colours of all glowing substances have the same starting point • They come from atoms and molecules that have been excited to states of energy. • Atoms in burning fireworks and stars have become excited by absorbing energy as heat; then convert that energy into coloured light which is emitted. • The colours shown by an atom depend on how it’s electrons are configured. • Thus, by investigating the colours an atoms of an element emit, we can determine its atomic structure and work out which element it is. ACTIVITY 1 ACTIVITY 2 Specific metal ions are used in fireworks to show different colours Metal ions can be identified by the unique colours of their flames. ACTIVITY 2 Specific metal ions are used in fireworks to show different colours Metal ions can be identified by the unique colours of their flames. HOW ARE THESE COLOURS EMITTED AT AN ATOMIC LEVEL? Meetal Kumar ATOMIC EMISSION SPECTRA in the ELECTROMAGNETIC SPECTRUM Electromagnetic spectrum: range of all wavelengths of electromagnetic radiation Atomic Emission Spectra: Set of wavelengths of the electromagnetic spectrum emitted by excited electrons of an atom What happens when white light passes through a prism? Instructions: 1) Turn on the ray box 2) Place the prism flat on the paper in front of the ray box so that the beam of light passes through it 3) Turn off the lights 4) Observe the beam of light as it enters and exits the prism What do u see? WHAT HAPPENS WHEN WHITE LIGHT PASSES THROUGH A PRISM? The light is separated and dispersed into the full visible spectrum FOR EXAMPLE: • Sunlight passes through a raindrop the light is dispersed into a visible rainbow QUESTION • What happens when we hold a prism in front of light emitted from these specific burning elements? Would we see the entire visible spectrum? • A: Yes, we would see the entire visible spectrum • B: No, we would see bands of light broken up • C: No, we would see the colours refracting back into its white light form • Answer: • B: No, we would see bands of light broken up For example, Na+ light is broken up into bands within the spectrum - Notice each element has a unique spectrum that is emitted, referred to as its ‘Fingerprint’ and allows us to determine which element is becoming excited FIRST LETS LOOK AT THE ELECTRON CONFIGURATION OF AN ATOM • Quantum numbers = energies of electrons in atoms or ‘shells’ • They are like the ‘address’ of the electron • No two electrons can occupy the same address • N is called Principal energy level • It is always a whole number QUESTION: • If these energy levels or ‘shells’ are not occupied by electrons, are the shells still present? Yes or no? • Answer: • YES! The energy levels are always present HOW DO FLAME TESTS WORK WHAT CAUSES THE ELECTRON TO BECOME EXCITED? • 1) Electron begins in Ground State (electron in n=1) • 2) Energy eg. heat is absorbed by electron • 3) Electron bounces to higher energy level enters Excited State (eg N=2,3,4) • 4) Electron releases Photon (light energy) moves back down to lower level energy • N=1 – UV rays ( not visible) • N=2 – Visible • N=3+ - IR rays (not visible) HOW DO FLAME TESTS WORK • Using all this information allows us to observe colours emitted from fireworks or outer space for example and determine which elements are becoming excited and emitting that colour of light EMISSION SPECTRA Pembe Hussain MARS Dark red patches Pale yellow patches CHEMICAL COMPOSITION OF MARS Iron oxide and small traces of calcium Pale yellow/white patches = hydrogen, helium, sulfur and sodium. JUPITER Pale grey Orange JUPITER’S COMPOSITION Hydrogen, helium with ammonium Phosurphous, sulfur and hydrocarbons SUN WHEN IN SPACE Naturally white with slight blue tinge SUN WHEN IN SPACE Helium and hydrogen COLOUR OF THE SUN WHEN IT IS UP DURING THE DAY SUN WHEN IN SPACE Helium and hydrogen No interfering wavelengths SO WHAT ARE WE TALKING ABOUT? VISIBLE LIGHT The only electromagnetic waves detectable by human eyes WHAT IS A WAVELENGTH? • It is from one “bump” to another. • The technical term for a “bump” is a crest Each colour has its own wavelength that sets it apart so we can see it WITHIN THE VISIBLE LIGHT SECTION, WE HAVE A BREAK DOWN OF COLOURS so that means: Visible light has its own spectrum` RIDDLE: What does a fingerprint belonging to a human being, and the electron configuration of an element have in common??? They are both unique to the individual and element Different electron configurations Different electrons being excited @ different energy levels (n) different photons released different wavelengths VISIBLE WAVELENGTH AND COLOUR SO: EACH ELEMENT HAS ITS OWN UNIQUE QUESTION: Does an element emits only one colour? Yes and no. We see only one colour but, as electrons travel between different energy levels, they release different photons and wavelengths. We just see the most dominant wavelength. POTASSIUM - K+ • Flame colour is lilac • YET: the potassium wavelength is a mixture of red @650 nm and blue @475 nm. 4000 A o 5000 6000 7000 An atom actually emits all of the colours, but the only colour detected by the human eye is that wavelength most dominant in the emission spectrum SO…. Potassium when heated emits a lilac flame because: It is the dominant wavelength 4000 A o 5000 6000 7000 EMISSION SPECTRUM shows the electron configuration of a specific atom EACH EMISSION SPECTRUM IS UNIQUE TO ON PARTICULAR ELEMENT 4000 A o 5000 6000 • Why? Because it is like a finger print 7000 APPLICATIONS: • Identify different chemicals in a solution/substance for art restoration and chemical analysis • Fireworks • Astronomers use telescopes with detection devices that are sensitive to wavelengths Determine composition FLAME TEST COLOURS Sodium, Na Calcium, Ca NEXT LESSON: • You will perform a first-hand investigation into observing the flame colours. You will be given unknown solutions and from this lesson, you will be expected to indentify them. FIREWORKS FIREWORKS HOW DO FLAME TESTS WORK