Chapter 12 Section 12.1
advertisement

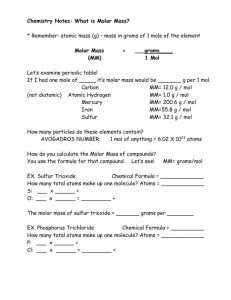
Section 12.1: Counting Particles of Matter Is it practical to count out 200 grains of rice, or 200 pieces of macaroni? No Way! We sell these items by the pound. How would we be able to calculate how many items are in a container without counting? There is! At your table, discuss a method to figure out how many items are in each bag, without counting. What information would you need? How many candy hearts are in the jar? What you need; the data: Mass of the empty jar (with stopper) = 209.624 g Mass of the full jar (with stopper)= 396.227 g Average mass of one candy heart= 1.92 g How many atoms are in 1.0079 g of Hydrogen if the average mass of one atom is 1.67425 x 10-24 g? What you need: Total mass • Mass of one atom What number do you get? 6.02 x 1023 atoms of H • The study of the relationships between the macroscopic quantities like mass and volume, and the submicroscopic quantities like number and mass of the atoms involved in a chemical reaction. Chemists cannot count individual items because the particles of matter are too small and too numerous- so we use this process. The group or unit of measure that we use in chemistry to count numbers of atoms, ions, molecules or formulas units of a substance (elements or compounds) Mole Map • http://www.youtube.com/watch?v=4QiGVqK7AS U • https://www.youtube.com/watch?v=TEl4jeETVm g&list=PLKEmXepzBsY9Zx4EHN27HHSQyvb4Xysb 4&index=6 6.02 x 1023 • • • This HUGE quantity represents the number of particles in one mole of ANY substance. It is just a number! A mole can be analogous to a dozen, a pair, a century…you know how many individual parts there are in each one of these measurements, right? 12, 2, 100…a mole is just A LOT more. Video The mass in grams of one mole of an element or a compound! (g/mol) For elements, it is the atomic mass in grams – One atom of magnesium = 24.31 u (atomic mass units ) – One mole of magnesium = 24.31 g/mol For compounds, it is the sum of all the masses of each element in the compound’s formula One mole MgSO4 = 24.31 + 32.07 + 4(16.0) = 120.4 g/mol These are not hard to calculate, because essentially the value of each is the same. It is the units that are different! • Formula Mass is a term used for IONIC compounds – NaCl has a FORMULA mass of 58.45 u and a MOLAR mass of 58.45 g/mol – ~ Same quantity, different unit Ex. CaCl2 Ca: 1 x 40.1 = 40.1 Cl: 2 x 35.5 = 71 Formula mass = 111.1 u Molar mass = 111.1 g/ mol • Molecular Mass is a term used for COVALENT compounds – C2H6 has a MOLECULAR mass of 30.1 u and a MOLAR mass of 30.1 g/mol Ex: C2H6O C: 2 x 12 = 24 H: 6 x 1 = 6 O: 1 x 16 = 16 Molecular mass = 46 u Molar mass = 46 g/ mol • • When solving problems like the following, you must use DIMENSIONAL ANALYSIS (Factor Label Method). Steps grams ↔ moles Molar mass ↔ “stuff” Avogadros # 68 g NH3 = ? mol 1 N = 1 x 14.007 = 14.007 g 3 H = 3 x 1.008 = 3.024 g 14.007 + 3.024= 17.031 g NH3 68 g NH3 x 1 mole NH3 = 3.99 mol NH3 17.031 g NH3 17.5 g CuO = ? mol 1 Cu = 1 x 63.546 = 63.546 g 1 O = 1 x 15.999 = 15.999 g 63.546 + 15.999 = 79.545 g CuO 17.5 g CuO x 1 mole CuO = 0.22 mol CuO 79.545 g CuO 0.550 mol F2 = ? g 2 F = 2 x 18.998 = 37.996 g 0.550 mol F2 x 37.996 g F2 1 mole F2 = 20.90 g F2 2.35 mol BaI2 = ? g 1 Ba = 1 x 137.33 = 137.33 g 2 I = 2 x 126.90 = 253.8 g 137.33 + 253.8 = 391.13 g 2.35 mol BaI2 x 391.13 g BaI2 = 919.16 g BaI2 1 mole BaI2 10.7 g Li = ? atoms 10.7 g Li x 1 mole Li X 6.02 x 1023 atoms Li 6.941 g Li 1 mole Li = 9.28 x 1023 atoms Li 144.6 g W = ? atoms 144.6 g W x 1 mole W X 6.02 x 1023 atoms W 183.85 g W 1 mole W = 4.73 x 1023 atoms W 1. Does 50.0 g S or 50.0 g Sn represent a greater # of atoms? 50.0 g S x 1 mole S X 6.02 x 1023 atoms S 32.066 g S 1 mole S = 9.39 x 1023 atoms S 50.0 g Sn x 1 mole Sn X 6.02 x 1023 atoms Sn 118.710 g Sn 1 mole Sn • = 2.54 x 1023 atoms Sn Sulfur is smaller- has a greater number of atoms What mass of water must be weighed to obtain 7.50 mol of H2O How many moles are in 17.5g copper (II) oxide How many molecules are in each sample? 89g nitrogen monoxide 10.8g boron trifluoride What is the molecular mass of aspirin (C9H8O4)