2012-2013-Northwest-Y2-Pharmacology-and-Development
advertisement

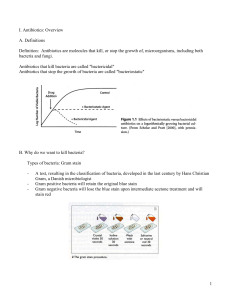
Pharmacology and development of Antibiotics (Penicillin) and Antiseptics 13/02/13 By: Mohit Kumar Sharma PhD Final year Cell types Eukaryotic cell Prokaryotic cell Bacterial classification based on Gram staining Gram staining technique Bacterial classification based on Gram staining Gram positive Gram negative Peptidoglycan Transpeptidase cross-linking to peptidoglycan sheets Few Examples of different classes of Bacteria Protection against bacteria - Skin - Tears - Saliva - Stomach acid Skin The skin is tough, dry, salty, oily, rich in fatty acids and urea, low in nutrients (lots of dead, empty cells) & thick. The sweat glands secrete a mixture of salt & fatty acids that inhibit many microbes. Tears Lysozyme is an enzyme, present in tears that LYSES many prokaryotic cells by digesting the peptidoglycan in their cell walls. - Through hydrolysis of glycosidic linkages. Saliva There is a continuous flow of fluid (saliva) through the mouth which FLUSHES loose microbes into the stomach. Stomach acid The stomach contains a strong (hydrochloric) acid. Many microbes are killed by this acidic environment and digested by the proteolytic enzymes in the digestive system. What are Antibiotics? - Antibiotics, also known as antibacterials, are types of medications that destroy or slow down the growth of bacteria. - The term antibiotic was first used in 1942 by Selman Waksman - The Greek word anti means "against", and bios means "life" (bacteria are life forms). - Many antibacterial compounds are relatively small molecules with a molecular weight of less than 2000 atomic mass units - The first antibiotic was penicillin. - Penicillin-related antibiotics such as ampicillin, amoxicillin benzylpenicilllin are widely used today to treat a variety of infections. and How do antibiotics work? Although there are a number of different types of antibiotic they all work in one of two ways: - A bactericidal antibiotic kills the bacteria. Penicillin is a bactericidal. A bactericidal usually either interferes with the formation of the bacterium's cell wall or its cell contents. - A bacteriostatic stops bacteria from multiplying. Targets of Antimicrobials 4. Protein synthes is inhibitors acting on ribosomes 1. Cell wall inhibitors Block synthesis and repair Penicillins Cephalosporins Carbapenems Vancomycin Bacitracin Fosfomycin Isoniazid Ribosome Erythromycin Clindamycin Synercid Pleuromutilins Substrate 2. Cell membrane Causelossofselective permeability Polymyxins Daptomycin Enzyme Product Site of action 30S subunit Aminoglycosides Gentamicin Streptomycin Tetracyclines Glycylcyclines Both 30S and 50S 3. DNA/RNA Inhibit replication and transcription Inhibit gyrase(unwinding enzyme) Quinolones Inhibit RNA polymerase Rifampin Site of action 50S subunit Blocks initiation of protein synthesis Linezolid mRNA DNA 5. Folic acid synthesis Block pathways and inhibit metabolism Sulfonamides (sulfa drugs) Trimethoprim Mechanisms of Drug Action Inhibition of cell wall synthesis Inhibition of nucleic acid structure and function Inhibition of protein synthesis Interference with cell membrane structure or function Inhibition of folic acid synthesis Penicillin History of Penicillin - 1928, Alexander Fleming - Observed antibiosis against bacteria by a fungus of the genus Penicillium. - 1940, Florey and Chain - Began work on isolating and synthezing large amounts of Penicillin. - 1940, Preclinical trials in mice - 1941, Phase II Clinical trial - 1944 - Used in WWII to treat infections - Late 1940’s - available for general use in US Alexander Fleming Antibiotic effect of the mold Penicillium chrysogenum Staphylococcus aureus (bacterium) Penicillium chrysogenum (fungus) Zone where bacterial growth is inhibited Penicillin Source: Penicillin chrysogenum Penicillin antibiotics grouped into generations based on spectrum of anti-microbial activity. (1st, 2nd , 3rd and 4th generations) Natural (penicillin G and V) Semisynthetic (Ampicillin, Carbenicillin) Classification Penicillins (penicillin G) – greatest activity against gram+, gram-cocci, non-beta-lactamase-producing anaerobes Antistaphylococcal penicillins (nafcillin) – resistant to staphylococcal beta-lactamases, active to staphylococci and streptococci Extended-spectrum penicillins (ampicillin) – retain antibacterial spectrum of penicillin with improved activity against gram- organisms, but are destroyed by beta-lactamases. Types of Penicillin Chemical Structure Penicillins as well as cephalosporins are called beta-lactam antibiotics, characterized by 3 fundamental structural requirements: the fused beta-lactam structure a free carboxyl acid group one or more substituted amino acid side chains Chemical structure of penicillins Structure Thiazolidine ring Beta-lactam ring Variable side chain (R group) - The R group is responsible for the activity of the drug and can be changed to improve activity of the antibiotic. - Cleavage of the beta-lactam ring will render the drug inactive.