Advanced Chemistry Chapter 9 Chemical Bonding Question Set #3
advertisement

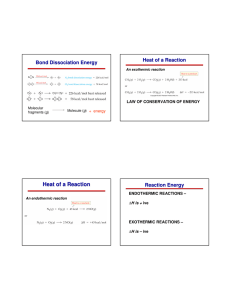
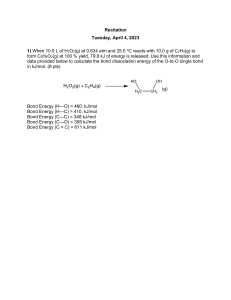
Advanced Chemistry Chapter 9 Chemical Bonding Question Set #3 9.55 Draw three resonance structures for the molecule N2O3 (atomic arrangement is ONNO2). Show the formal charges. 9.56 Draw three reasonable resonance structures for the OCN- ion. Show formal charges. 9.61 The AlI3 (aluminum triiodide) has an incomplete octet around Al. Draw three resonance structures of the molecule in which the octet rule is satisfied for both the Al and the I atoms. Show formal charges. 9.63 Of the noble gases only Kr, Xe, and Rn are known to form a few compounds with O and.or F. Write Lewis structures for the following molecules; (In each case Xe is the central atom.) a. XeF2 b. XeF4 c. XeF6 d. XeO2F2 9.64 Write the Lewis structure for SbCl5. Does this molecule obey the octet rule? 9.69 From the following data, calculate the average bond enthalpy for the N-H bond: NH3(g) → NH2(g) + H(g) ∆H⁰ = 435 kJ/mol NH2(g) → NH(g) + H(g) ∆H⁰ = 381 kJ/mol NH(g) → N(g) + H(g) ∆H⁰ = 360 kJ/mol 9.70 For the reaction O(g) + O2(g) → O3(g) ∆H⁰ = -107.2 kJ/mol Calculate the average bond enthalpy in O3. 9.71 The bond enthalpy of F2(g) is 156.9 kJ/mol. Calculate ∆H⁰f for F(g). 1 2