law
advertisement

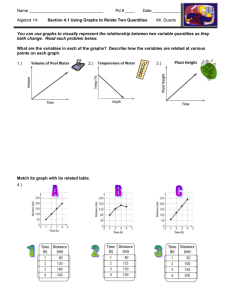
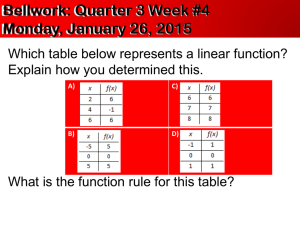
What is “Science?” Why do we really need to study it? Field and lab investigations • Science is a process • We begin with a question, and seek an answer by investigating the natural world • We use a variety of methods to investigate – Conduct experiments – Work of other scientists • We use tools (lab equipment) to help us find the answers we seek What correlation do the following have with concerning ourselves with “lab safety?” • Falling down the stairs • Slipping on water • Getting robbed THAT BAD THINGS CAN HAPPEN AT ANY TIME AND WE WANT TO PREVENT THOSE THINGS FROM HAPPENING IN LAB. • • • • • Never eat or drink in the lab. Remain alert at all times. Keep your area free of clutter. Follow both written and verbal directions. Unauthorized experiments can be dangerous. • Report any accident, incident, or hazard to me immediately, even if you think it’s “no big deal.” • Students may not work alone. • Remain at your workstation. The world needs more Lerts! • Always wear goggles and other safety items. • If you get any chemical in your eyes, immediately flush your eyes (including under your eyelids) with water at the eyewash station for at least 15 minutes. Notify your teacher. • Secure all loose items. • Hair should be pulled back. • Contacts are now allowed. • Know where to find the safety equipment. Where are the following pieces of safety equipment found in our room? • • • • Fire extinguisher Safety shower Eye wash Fire blanket What are each of them used for? • • • • Fire extinguisher Safety shower Eye wash Fire blanket • To put out a fire if your clothes are on fire • To put out a fire in the room (i.e. paper) • To use if you get something hazardous on your body • To use if you get something harmful in your eye(s) Blood and Body Fluids Safety • Wear gloves when someone is bleeding or vomiting • Clean everything touched by blood or vomit with a disinfectant • Remove your latex gloves by pulling them off inside out • All materials used to clean up blood or other possible infectious materials should be disposed of in the proper biohazardous waste bag • Standard Biohazardous waste bags are red with the biohazardous waste symbol on them. If one is not available, use a regular plastic garbage bag and attach biohazardous waste sticker to the bag • Wash your hands thoroughly with a disinfectant when you are finished • • • • • • • • • • Never pipette by mouth. Throw away broken or chipped glassware Hot glass looks like cool glass. Hot glass does not go in water. Point test tubes away from people when heating them. Always pour acid into water. Touch electrical devices only with dry hands. Smell a chemical by waving your hand over it and letting the smell drift to your nose. Don’t pour chemicals down the sink. Never return unused chemicals to the original container. What do the following symbols represent? Safety Warning Symbols Safety Warning Symbols Cool! Oops, I was supposed to read the lab directions first!!!! What does M.S.D.S. stand for? Why do we use them during lab? • Material Safety Data Sheets • It provides information on the safe use, handling, storing, and potential hazards of a chemical. Examples of why it’s important to know what types of chemicals you’re dealing with and how to store them: • Generation of heat (acids & bases) • Violent reaction (acrolein & acids or other catalyst) • Formation of toxic vapors or gases (cyanide salt & acid) • Formation of a flammable gas (alkali metal & water) • Fire or Explosion (perchloric acid & acetic anhydride) Safety Terms: Caustic or corrosive- Will corrode or eat away metal, skin, or other substances. Volatile- Evaporates quickly, may form dangerous vapors. Flammable (may ignite)will catch on fire or explode easily. Ventilation- removing contaminated air and brining in fresh air. Where have you seen the following sign before? NFPA Hazard Diamond • Inside/outside of labs • On chemical bottles • On trucks (gases, different chemicals) • Hair salons Flammability Hazard Health Hazard All chemicals must be labeled, and the label must include the NFPA hazard diamond Stability Hazard Special Information Risk Level 0 Minimal 1 Slight 2 Moderate 3 Severe 4 Extreme How dangerous is this one? Extremely flammable Moderately unstable Not a health risk Keep away from water 4 0 2 W •Extinguishers must be located and marked so they are easily seen in a laboratory •The extinguisher must be inspected annually and be tagged with the inspection history www.hometrainingtools.com/ tbimages/11950.lg.jpg Triple Beam Balance • To measure the mass of substances or objects accurately • Mass is the amount of matter in an object. Graduated cylinder • To measure volume of liquids accurately to within about 1%. • They are for general purpose use, but not for quantitative analysis. If greater accuracy is needed, use a pipette or volumetric flask. • The guard is to protect it from breaking if it tips over The Meniscus—reading volume correctly • When water is placed in a glass or plastic container the surface takes on a curved shape. • This curve is known as a meniscus. Volumetric glassware is calibrated so that reading the bottom of the meniscus viewed at eye level gives accurate results. • Viewing the meniscus at any other angle will give inaccurate results. SI Ruler (“Metric” is slang.) • To measure the lengths of solid objects accurately SI thermometer • To measure temperature accurately • Metric units are degrees Celsius, oC www.civilization.ca/.../ images/memorabilia3.jpg Magnifying glass (hand lens) • To make objects appear larger than they are Bunsen burner • To heat substances (with a flame) Hot plate • To heat substances using electricity, not a flame • This is usually safer when heating chemicals Ring stand with clamp and ring • To hold containers away from a heat source during an experiment Safety goggles • To protect eyes against burns, cuts, or flying objects Microscope • To view specimens that are too small to see with the naked eye Compound microscope Eyepiece Body tube Revolving nosepiece Low power objective Coarse adjustment (focus) Fine adjustment (focus) Arm Stage Clip High power objective Stage Mirror (or light source) Diaphragm (controls amount of light) Base http://www.cat.cc.md.us/courses/bio141/labmanua/intro/stageslide.html http://www.laboratory-supply.com/images/slidefrosted.jpg Microscope Slide • To hold a specimen (sample) for viewing through a microscope http://www.scitoys.com/scitoys/scitoys/thermo/liquid_metal/liquid_metal.html Cover slip • To cover a specimen (or sample) on a slide Medicine dropper (Eyedropper) • To drop small amount of liquids http://www.dryeye.org/products.htm www.emsdiasum.com/.../ tweezers/images2/72991.jpg Forceps • To pick up small items that you should not touch Petri dish • To grow bacteria and other tiny living things Test tube • To hold liquids and chemicals during experiments (investigations) Test tube rack • To hold test tubes during an investigation. (After cleaning, test tubes can be placed upside down to dry.) http://www.delta-education.com/images/products/2000855.jpg Test tube clamp • To hold test tubes over heat or away from your body Erlenmeyer flask • Used for mixing, transporting, and reacting, but not for accurate measurements. • The volumes stamped on the sides are approximate. www.secure.sciencecompany.com/.../ nc6282n.jpg Florence flask • Used for mixing, transporting, and reacting, but not for accurate measurements. • The volumes stamped on the sides are approximate. Beaker • Used for mixing, transporting, and reacting, but not for accurate measurements. • The volumes stamped on the sides are approximate Tongs • To pick up large objects that you should not touch (e.g., hot containers, flasks of acids, etc.) Funnel To pour liquids or powders from one container to another without spilling anything Stirring rod To mix chemicals and hot liquids together by stirring Accurate measurement When measuring distance on the TAKS •You will have a ruler on the side of your formula sheet. •USE IT if you are asked to measure distance on a map or a length. Measure the length of a tile. Measure the width of your folder. What is the volume of liquid in this graduated cylinder? 29 mL When taking the mass of solids •Use a mass boat or paper. •Subtract out the mass of the container or paper to zero the balance. •Clean up spills so they don’t corrode the balance. Reading Graphs and Making Calculations •Scientists must analyze graphs to understand an experiment’s result. • The Dependent variable is on the Yaxis. •The Independent Variable on a graph is on the X-axis. Pie Graphs • Pie graphs are used to show how a whole is broken up into its parts. • Note that parts should add up to 100% when values are given Bar Graphs • Bar graphs are used to compare measurements taken from a number of objects or categories. • They demonstrate trends in data Line Graphs • Most graphs show the relationship between two variables. • The lines are usually drawn either straight or curved. • These "smoothed" lines do not have to touch all the data points, but they should at least get close to most of them. They are called bestfit lines Pictographs Pictographs use symbols to represent numbers. Always check keys and legends to accurately read units and labels on graphs. Nutrition Labels • A U.S. government agency defines what information must appear on a food label. • The meaning of terms such as “low fat” are strictly defined for use on labels – If a label claims that the product is unsweetened or that there is no sugar added, it means no common table sugar (sucrose) has been added. – It does NOT mean there are no other sugars present • Info is based on a single serving – Careful, single servings are sometimes chosen so that the numbers “look good” at a glance check this when comparing two labels – For example, an individual bag of chips may have three servings, rather than one •You may be asked to calculate a % from a label. •To find a %, divide the # of items you have by the total #, then multiply by 100. Analyzing Product Ads (consumer info) • Every day, companies try to sell you their products. • Television commercials and newspaper and magazine ads tell you why certain products are best. • You must use your critical-thinking and problemsolving skills to decide for yourself if the ads are correct. – Remember—their motive is $$$ so they may “shade” the truth to convince you; you have to outsmart them! Feel Better Fast with Vitaplex!--Really? • The word “scientific” is to make you believe this is factual • It’s true that your body needs protein to survive and that your body converts protein into amino acids • It is NOT true that excess protein will not be converted into fat • Vitamins are essential to good health, but they do not provide energy • Excess vitamins are excreted or stored in the fat (too much stored in fat can make you ill) • So, will Vitaplex give you energy and make you feel better? Scientific Process • Prentice Hall – Chapter 1: Section 2 – Pages 8-15 Scientific Process • Why does it rain? Give an answer without using a scientific explanation. • Suppose someone does not believe your explanation. Could you supply evidence to support your explanation? Steps of the Scientific Method • Purpose – Ask a question • Form a Hypothesis • Experimentation – Set up a controlled experiment • Record and Analyze data • Draw a Conclusion Scientific Method • • • • What is it? A method of investigating questions Why do we need it? We must make decisions based on what we know and observe NOT what we want it to be! • Does it apply to us outside of the lab/classroom? • Yes, of course! You use it all the time and probably do not even realize it. Scientific Method Results may require adjusting hypothesis Observations Hypothesis Experiments Theory Cycle repeated until the hypothesis fits all observed experimental facts Observations Explains results Repeated successful experiments Law Describes natural phenomena Theory vs. Law • What is the difference between a theory and a law? • Theory – well tested hypotheses proven beyond reasonable doubt (however, they can be disproven) • Law – is a statement of fact meant to explain a set of actions Theory vs. Law • Theory – explains nature. • They answer the question: WHY does that happen? • Law – describes nature. • They answer the question: WHAT will happen? • Laws and theories are different kinds of things. Theories do not become laws. Laws do not become theories. Ex: Lions do not become tigers. Experimentation • Experimental group • Receives some kind of treatment or condition (plant given miracle grow) • Control group • Receives no treatment, used to compare results (plant not given miracle grow) Why do we need a control group? • To determine the actual affects that the factors you are testing have on that group Types of Variables • Independent Variable (Manipulated variable) • The factor you change, what you do to your group (Miracle Grow) • Dependent Variable (Responding variable) • What happens as a result of that treatment, what you are measuring (Growth of plant)