History of Stats at the FDA - American Statistical Association
advertisement

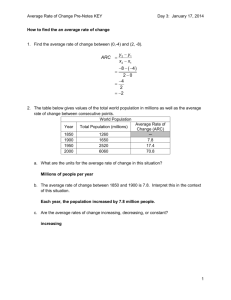
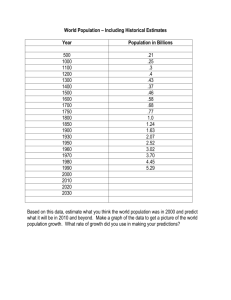
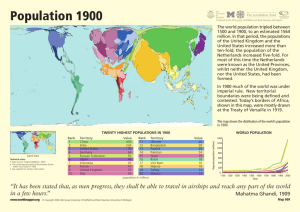
A History of Stats at the FDA Mary A. Foulkes, Ph.D. U.S. Food And Drug Administration Office of Biostatistics and Epidemiology Center for Biologics Evaluation and Research 2006 FDA/Industry Statistics Workshop The views presented are my own and do not represent the official view of the U.S. Food and Drug Administration FDA Centers • Center for Biological Evaluation and Research (CBER) • Center for Drug Evaluation and Research (CDER) • Center for Device and Radiological Health (CDRH) • Center for Food Safety and Applied Nutrition (CFSAN) • Center for Veterinary Medicine (CVM) • National Center for Toxicological Research (NCTR) OUTLINE • • • • • • Early years Kefauver-Harris Amendments Grandfather Women, Kids and Animals Globalization Critical Path and Beyond A Public Outcry for a New Law • Upton Sinclair’s book, The Jungle, drew attention to adulterated meat • Meat sales dropped by 1/3 • Roosevelt was persuaded to sign Pure Food and Drugs Act on June 30, 1906 along with the Meat Inspection Act • 1906 Act transformed a scientific bureau into a regulatory agency that would become FDA Pre-1962 • Safety • Advertising An original 1-gallon bottle of Elixir Sulfanilamide Wax, P. M. Ann Intern Med 1995;122:456-461 Public Health Reports, January/February 2000, Volume 115 Pre-1962 • • • • • Safety Advertising Elixir Sulfanilamide LD 50 Bioassay Kefauver-Harris Amendments Evidence consisting of adequate and well-controlled investigations, including clinical investigations, by experts qualified by scientific training and experience to evaluate the effectiveness of the drug involved, on the basis of which it could fairly and responsibly be concluded by such experts that the drug will have the effect it purports or is represented to have under the conditions of use prescribed, recommended, or suggested in the labeling or proposed labeling thereof. FD & C Act Section 505(d) Kefauver-Harris Amendments Evidence consisting of adequate and well-controlled investigations, including clinical investigations, by experts qualified by scientific training and experience to evaluate the effectiveness of the drug involved, on the basis of which it could fairly and responsibly be concluded by such experts that the drug will have the effect it purports or is represented to have under the conditions of use prescribed, recommended, or suggested in the labeling or proposed labeling thereof. FD & C Act Section 505(d) 1962 and Beyond • DESI (1938 – 1962) drugs – Grandfathered • Fed Reg 1970 – “appropriate statistical methods” • Orphan Drug Act 1983 • 1990’s – Expanding demographics (Age, Gender) • FDAMA 1997 FDA Biometry and Epidemiology Methodology Advisory Committee The American Statistician, 1968 JASA, 1999 • Treatment of insomnia - triazolam • Re-analysis of 25 plcbo-contr trials • Mixed-effects regression models – using all available data • Diff btw trials and spontaneous reports – Recommend: longer term, high dose studies A priori Analysis Plan “Still, it is an error to argue in front of your data. You find yourself insensibly twisting them around to fit your theory.” Sherlock Holmes in The Adventure of Wisteria Lodge Control of Type I error • • • • • Primary and secondary outcomes Composite endpoints Power Essential Multiplicity Implications for design Subgroups • • • • Age enrollment to match indication Gender Demographics Pediatric Rule Other Issues • • • • • • • Active/placebo/historical controls Adaptive trials Combination products Adverse Events/MedDRA/Data Mining Multiplicity Endpoints QA/QC Group Sequential Boundaries Interim Monitoring • • • • • Outcome trials Serious morbidity/mortality Minimize risks Futility Regulatory implications Large Safety Studies • • • • • Ibuprofen CLASS VIGOR Rotavirus SMART N N N N N = = = = = 84,192 8059 8076 68,038 26,355 (1995) (2000) (2000) (2006) (2006) Counterterrorism “Animal Rule” “Evidence Needed to Demonstrate Effectiveness of New Drugs When Human Efficacy Studies are not Ethical or Feasible” Counterterrorism Under Animal Rule, use data from two species to predict human responses: • • • • • • Anthrax Botulism Plague Smallpox Tularemia Viral hemorrhagic fevers ICH • International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use • Regions: EU, Japan, US • Observers: WHO, others • Co-sponsors: EC, UFPIA, MHW, JPMA, FDA, PhRMA Int’l Conf on Harmonisation • • • • • • E-3 Clinical Study Reports E-5 Acceptance of foreign data E-6 Good Clinical Practice E-8 Gen’l Consid’s for Clinical Trials E-9 Statistical Principles E-10 Choice of control groups Critical Path and Beyond • • • • • • Design efficiency Conduct efficiency Targetted therapies Imputation Simulation Extrapolation Critical Path and Beyond Basic Research Prototype Design or Discovery Preclinical Development Clinical Development Market Application Critical Path FDA Filing/Approval & Launch Preparation Approval Critical Path and Beyond • • • • • • Improved trial efficiency Better prospective planning Use of prior information Handling of missing data Analysis of multiple endpoints Addressing non-inferiority “Random Sample” of recent FDA Statisticians Celebrating 100 Years of Public Service Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions 1900 1950 2000 Year of Statistical Contributions 2010 Pace of Statistical Contributions The Sky is the Limit 1900 1950 2000 Year of Statistical Contributions 2010