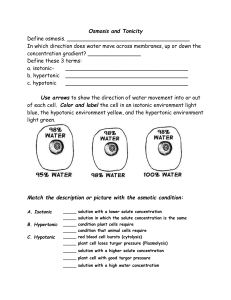
Plasma Membrane
advertisement

Break into your lab groups and look at the 5 choose your transport cards on your lab table. Identify if the card is an example of: Diffusion, Osmosis or Active Transport Read pages 76 and 77 of the Holt textbook and use the information to create the tri-fold described on the next slide. On the top of each tab, write a description of what is happening to the cell if placed in that particular environment. On the bottom of the tab, draw a picture of how water is moving in and out of the cell. 10% NaCl 90%H2O 10% NaCl 90%H2O NO NET MOVEMENT cell stays the same 10% NaCl 90%H2O 20% NaCl 80%H2O More water moves into the than leaves the cell causing it to swell. 15% NaCl 85%H2O 5% NaCl 95%H2O More water moves out of the cell than into the cell causing it to shrink. An osmosis investigation was conducted using chicken eggs to represent cells with semipermeable membranes. The mass of each egg was measured to determine how much water diffused into or out of the eggs. The eggs were first soaked in vinegar to dissolve the shell. Each egg was then placed in one of three different solutions for 24 hours. Corn Syrup 5% water Vinegar 85% water Distilled Water 100% water Based on your knowledge of osmosis and the way water molecules move in various solutions, what would happen if you took a fish from the ocean (salt water) and placed it in a fresh water lake? Because it would be going from an environment high in solute to an environment much lower in solute, the fish would swell and die. What is an Isotonic Solution? In an isotonic solution, the concentration of dissolved substances in the solution is the same as the concentration of dissolved substances inside the cell. What happens to a cell when placed in an isotonic Solution? The cell remains unchanged What is an Hypotonic Solution? In an hypotonic solution the concentration of dissolved substances is lower in the solution outside the cell than the concentration inside the cell. What happens to a cell when placed in an Hypotonic Solution? The cell will swell What is an Hypertonic Solution? In an hypertonic solution the concentration of dissolved substance outside the cell is higher than the concentration inside the cell. What happens to a cell when placed in an Hypertonic Solution? The cell will shrink