Osmosis & Tonicity Worksheet: Real-Life Applications
advertisement

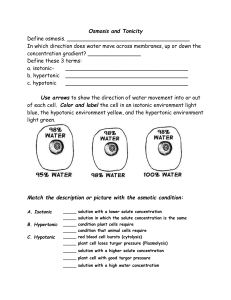
Bio 12 pg 74-75 Name: __________________ Critical Thinking Qs: Date: __________________ Applying Osmosis and Tonicity to Real Life! Block: ______ Helpful Hints to answering the Qs below: -tonicity Qs involve comparing the concentration of solutes in 2 different solutions -hypotonic, hypertonic and isotonic are terms to use while comparing two solutions, you must specify hypotonic compared to _________ ie) the solution “A” is hypotonic compared to the cell “B” tells us there are less solutes in solution A compared to the inside of cell B -osmosis involves the diffusion of water moves from HIGH to low concentration -water will always move from a hypotonic condition TO the more hypertonic condition Be sure to use the terms hypotonic, isotonic, hypertonic, osmosis, equilibrium & concentration gradient as appropriate in each of your answers. 1. During cold temperatures, roads are sometimes salted to melt ice. As a result of the salt, many of the roadside plants die of dehydration. Explain why using the key vocab. 2. Why do grocery store owners spray fresh fruits and vegetables with water? What causes carrots forgotten in the fridge to go limp? 3. If a shipwrecked crew drinks sea water, they will probably die. Why? 4. Contact lenses are worn overtop of the cornea to help improve vision. a) Why do your eyes feel dry and sore after wearing contacts for a long time? b) Why must contact lenses be cleaned / stored overnight with saline solution and NOT water? 5. Your friend tries on your favorite ring but 5 minutes later, her finger has become so swollen that she can’t get the ring off.What type of solution can be used to safely remove the ring and how? Apply your knowledge of osmosis and tonicity! 6. Normal human body tonicity is determined by the total solute concentration (salts and proteins mainly) in the fluid and water intake/loss. Why is it that spending too long in an hot tub, makes your fingers “pruny”? Why don’t you burst completely? 7. An experiment was conducted to determine the concentration of molecules in the cytoplasm of potato cells. Five potato discs were each put in 5 different test tubes with different concentrations of sugar solutions shown below: Concentration Test Initial Potato Mass Final Potato Change in of Sugar Tube (grams) Mass (grams) Mass (%) Solution (%) 1 30.0 5.0 4.0 -20 2 20.0 4.8 7.3 -10 3 10.0 5.2 5.5 +6 4 5.0 4.7 5.4 +15 0.0 5 5.1 6.1 +20 (distilled water) a) Name and describe the process that allowed the potato cells to gain and lose mass when placed in the sugar solution. Process Name: ________________ Description: ______________________________________________ b) Explain the change in mass of the potato disc in test tube 1. c) Explain the change in mass of the potato disc in test tube 4 d) What concentration of sugar solution is closest to an isotonic solution compared to the potato?