Benzylidene Acetal Synthesis: Organic Chemistry Lab Experiment
advertisement

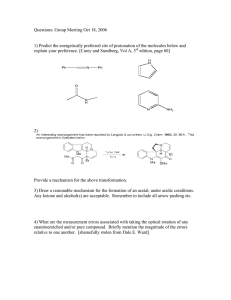
IN-PERSON EXPERIMENT 1 Synthesis of Benzylidene Acetal Introduction Organic chemistry is a powerful toolbox of reactions that allow chemists to take simple building blocks and assemble complex molecules. Due to the vast array of organic reactions, organic chemists can synthesize virtually any molecule that comes to their mind. One of the most challenging fields in organic chemistry is the total synthesis of natural products (i.e. , the replication of molecules that are produced by the living organisms that surround us) . Humans have discovered innumerous examples of natural products with promising applications for the production of cosmetics, food, medicines, textiles, etc.; however, plants and other organisms may only produce very small quantities of the natural product that we wish to isolate. Thus, organic chemists need to find synthetic ways to construct these very complicated molecules. Nature is very effective at constructing complex molecules thanks to the selectivity of the proteins involved in their production. On the contrary, chemists are not as efficient at synthesizing complicated molecules due to the large number of functional groups contained within these compounds. This results from the fact chat chemists wish to perform a specific reaction at only one functional group; however, there may be other sites within these molecules where a reaction can also take place. One strategy to aid an organic chemist in obtaining selective reactions is through the use of ''protecting groups." The introduction of a protecting group can be done to: (1) block a functional gro up from undergoing a chemical reaction (e.g., reduction of an ester within a molecule that also contains a ketone); or (2) prevent a functional rou . C . 'h . ( C . G' g p £rom mtenenng wit a reaction e.g., perrormmg a ngnard reaction with a molecule containing an alcohol) . Ideally, chemists prefer to have protecting groups that will not be removed during the reaction that they wish to perform but are easily introduced and removed afterwards to once again obtain the original functional group. A good real-world an~og~e for the use of pro_tecting ~rours in organic chemistry is the employment of pamter s tape by a house paint~r. Pamte~ s tape allows a painter to easily mask the parts of a room that they do not wish to paint (e.g., baseboards, window frames, electrical plugs) but then simply remove the tape once they have completed painting to obtain their finished project. Functional groups such as alcohols, amines, and carbonyls can require protection in mulcistep syntheses to ensure that they are not converted to Lmdesirable groups or to 1 NOTES . . d fi aki lace. Common protectmake sure that the desired reacnon is not prevente rom t ng P . d (3) tals c al h l . (2) carbamates for amines; an ace ing groups include: (1) silyl eth ers ror co o s, and ketals for carbonyls. \ I /Si,CI R/'--..OH NEt3 Alcohol 0 R \ I /'---.. ,.Si, 0 Silyl Ether 0 Xo)lo)lo>( 0 R✓"NH2 R✓-"N)lO>( Amine Carbamate H 0 R1 )l HO ~OH R2 (7 oyo R1 R2 Carbonyl Acetal For R1 and R2 = H, alkyl, aryl Scheme 1-1. Common protecting groups used to conserve alcohol, amine, and carbonyl substrates. In this experiment, we will investigate the protection of an aldehyde substrate through the formation of an acetal. In general, the formation of an acetal involves the reaction between an aldehyde with two hydroxyl groups under acidic conditions. The mechanism for this transformation involves the protonation of the carbonyl group (to render it more electrophilic) and arrack by a hydroxyl group to form a hemiacetal. Subsequent protonation of the hemiacetal and nucleophilic attack by a second hydroxyl group yields the acetal produce. Acetals can be formed using two equivalents of an alcohol or one equivalent of a compound containing two hydroxyl groups (also known as diol). Diols are more often used when protecting carbonyl substrates as cyclic acetals are formed and these acetals provide an ideal balance between stability under reaction conditions and ease of removal once the protecting group is no longer needed. Cyclic acecals are also of interest to our course as these compounds form six-membered rings similar to those found in cyclohexane or carbohydrate molecules. Cyclohexyl rings have non-planar configurations and arrange themselves in three dimensions to minimize the overall energy of the molecule. Six-membered rings can adopt a variety of conformational isomers, such as the chair and boat conformers, to orient into the most stable structure. Furthermore, substituents on cyclohexyl rings can occupy either the axial or 2 equatorial positions within the rings. As such, it is possible to h ave different stereoisomers form depending on which positions the substituents are fo und in . The most stable structures are those where the bulky substituents occupy the equatorial position to reduce the amow1t of steric hindrance within the molecule. NOTES Objective In this experiment, you will prepare a benzylidene acetal through the reaction of benzaldehyde and 1, 1, 1-tris(hydroxymethyl)ethane under acid conditions. You will determine th e composition of your product using thin layer chromatography (TLC) and nuclear m agnetic resonance (NMR) spectroscopy. 0 (i'H oyoH (i'o References D. Klein, Organic Chemistry: 3rd Edition, John W iley and Sons, H oboken, 2017, pp. 844-897 (Chapters 4 and 19) Pre- lab Preparation Besides preparing your lab report as outlined in the Laboratory Information section, familiarize yourself with filtration, thin layer chromatograp hy (see the Laboratory Techniques section and videos posted to Avenue), NMR Sp ectroscopy (see the Spectroscopy section and video posted to Avenue), and the safety info rmation fo r the chemicals that will be used in this experiment (Table 1-1) . Also answer the following questions: 1. The synthesis of an acetal is a reversible process and as such its preparation typically involves t!1e removal of water to obtain high acetal product yields. Explain why the reaction performed in this experiment can take place using water as a solvent. HinesWhat chemical principle governs product for mation in this reaction ? What is the physical state of the acetal formed product? W hat is the solubility of the acetal product? 2. D raw all possible stereoisomers of the benzylidene acetal product formed in this experiment. Use the following template and assume that each stereoisomer adopts the chair con fo rmer. Further, rank the stabilities of the stereoisomers from MOST to LEAST stable. In- Person Experim ent 1 Synthesis of Benzylidene Acetal ----liiiiiiiiiiiii;;;;;;;;;;;;;;:;;;;;;,___.;::=-----:= 3 NOTES 3. The crude benzylidene acetal product is purified via filtration (step 6) and crystallization (steps c to e) as outlined in the experimental procedure. Explain how these steps allow for the purification of benzylidene acetal. Be sure to outline which reaction component(s) is/are being removed during the filtration (step 6) and crystallization (steps c to e) procedures. 4. Acetals are also commonly used to protect 1,2- and 1,3-diol moieties in carbohydrate subsuates. Protection of 1,2-diols is achieved using acetone, while 1,3-diols are protected using benzaldehyde. Draw the acetal products for the following two reactions: 0 0 OH A \ ~OH [W] OH ? OH Ph)lH \ A AJ [WJ . ? 1,3-diol 1,2-diol The protection of a 1,3-diol is not typically accomplished using acetone. Draw the product formed when a 1,3-diol is reacted with acetone and propose an explanation as to why these acetals are not favoured for the protection of 1,3-diols. Table 1-1. Safety information for the chemicals employed in Experiment 1. COM POUND Acetone Benzaldehyde Sodium sulfate Petroleum ether HAZARD STATEMENTS ¢ ~ ¢ ~ ¢ ¢ ~ ~~ Harmful if swallowed or inhaled Causes skin or eye irritation Highly flammable liquid and vapours Harmful if swallowed or inhaled Causes skin or eye irritation Toxic to aquatic life Harmful if swallowed or inhaled Causes skin or eye irritation May be fatal if swallowed Causes skin or eye irritation May cause drowsiness or dizziness May cause damage to organs through prolonged or repeated exposure if inhaled Toxic to aquatic life Highly flammable liquid and vapours 1,1,1-tris( hyd roxym ethyl)eth an e 4 ¢ Causes skin or eye irritation NOTES HAZARD STATEMENTS COMPOUND Sulfuric acid (concentrated) \ Tol"'ne ~ 0 0 ~ ~ Harmful if swallowed or inhaled causes serious skin burns or eye damage May be fatal if swallowed causes skin or eye irritation May cause drowsiness or dizziness May cause damage to organs through prolonged or repeated exposure if inhaled Highly flammable liquid and vapours Experimental Procedure a. First, rinse a 10 mL round-bottom vial and magnetic stir bar with acetone and allow them to dry. To the vial add 1.1 g of 1, 1,1-tris(hydroxymethyl)ethane, 2.5 mL of water and - 10 drops of concentrated sulfuric acid. Stir the reaction mixture at room temperature until all of the solids have dissolved (-5 min). If necessary, use the vortex mixer to aid in the dissolution of 1, 1, 1-tris(hydroxymethyl)ethane. Once all of the solids have dissolved, add 0.56 mL of benzaldehyde to your reaction mixture and stir VIGOROUSLY for - 15 min. Procedure Note: Benzaldehyde is NOT soluble in water and thus the reaction needs to be stirred VIGOROUSLY to ensure that the reaction occurs! b. After allowing your reaction to stir for at least 15 min, add a couple of small pieces of ice to the vial. Continue to vigorously stir the mixture. Once a sol id begins to precipitate from solution, place the vial into an ice bath for 5-10 min. Collect the product by vacuum filtration using a Hirsch funnel and wash the product with three portions (-2 mL) of water. Procedure Note: If the solid product does not form, check the pH of your solution. The pH of the solution should be< 2. If not, add a few more drops of sulfuric acid and continue to vigorously stir your reaction for another 10 min. c. Place your crude product in_ a 25 mL flask and add - 10 mL of toluene. Gently heat, but do NOT boil your solution. If you observe two layers in your solution, use a glass pipette to remove the aqueous (bottom) layer. Then dry your organic solution by adding a spatula tip of Na2SO 4 and allowing the solution to stand for -5 min to absorb any water that is present. In - Person Experiment 1 Synthesis of Benzylidene Acetal 5 d. Decant the solution directly into a 30 mL beaker containing 10 mL of petroleum ether. Ensure that Na 2SO 4 is NOT transferred into the beaker. Allow the reaction mixture co stand in an ice bath for - 5 min. e. Collect the product by vacuum filtration using a Hirsch funnel. Wash the product with three portions (- 2 mL) of petroleum ether. Dry the product by drawing air through the funnel for a few minutes. Record the weight of the product. f Perform a thin layer chromatographic (TLC) analysis of your pure product. First, dissolve -25% of your product in 1-2 mL of acetone. Obtain a chromatography jar and add 5 mL of the TLC solvent mixture (70:30 petroleum echer:echyl acetate) to the jar. You can share a TLC developing chamber with your fumehood partner. Obtain 1 TLC place and 1 capillary cube. Prepare your TLC plate by spotting only your pure product (no reference samples). Develop your TLC plate and visualize your analysis using the UV lamp. Draw an illustration of your TLC plate (to scale) in your Jab notebook and calculate the J¼value for each spot. Using the information provided by your TA, clearly identify and label each sample in your TLC analysis. g. Determine the product yield. h. Assign the 1H and 13C NMR spectra of your product. 6