Acetal Formation: Mechanism, Protection, and Storage
advertisement
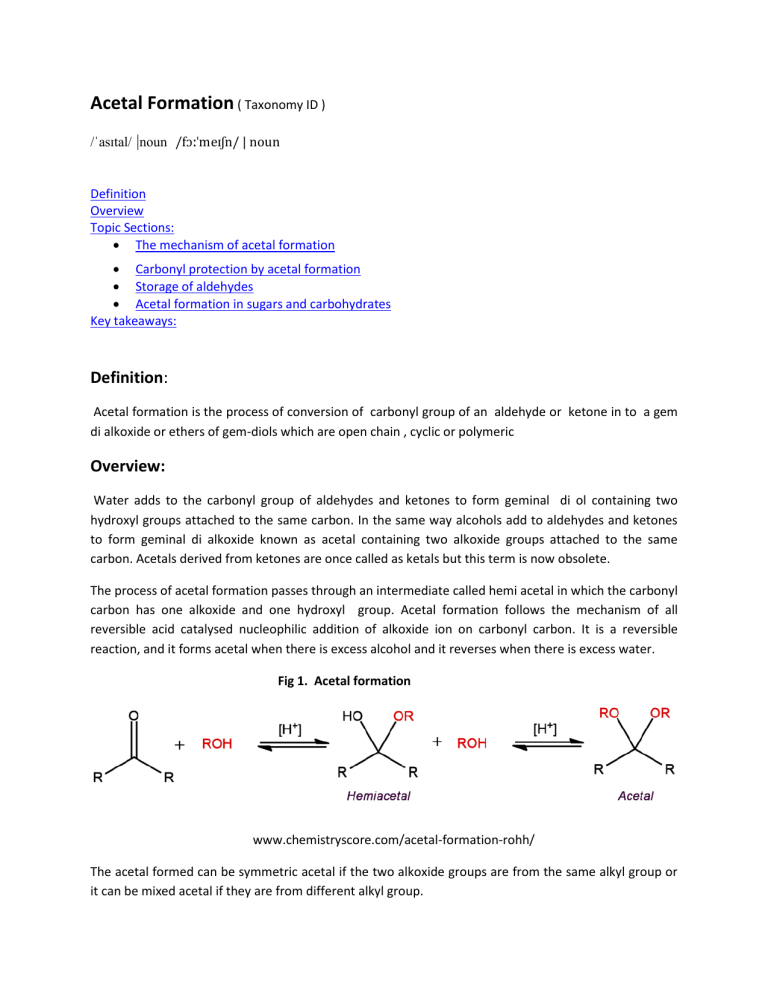
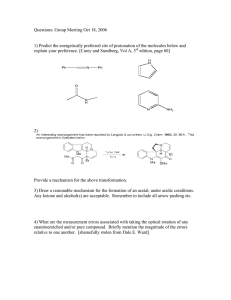
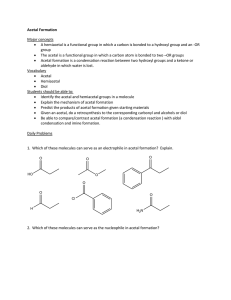
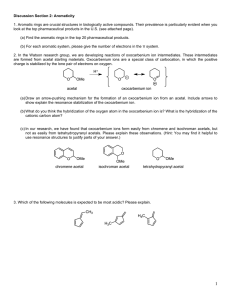
Acetal Formation ( Taxonomy ID ) /ˈasɪtal/ |noun /fɔːˈmeɪʃn/ | noun Definition Overview Topic Sections: The mechanism of acetal formation Carbonyl protection by acetal formation Storage of aldehydes Acetal formation in sugars and carbohydrates Key takeaways: Definition: Acetal formation is the process of conversion of carbonyl group of an aldehyde or ketone in to a gem di alkoxide or ethers of gem-diols which are open chain , cyclic or polymeric Overview: Water adds to the carbonyl group of aldehydes and ketones to form geminal di ol containing two hydroxyl groups attached to the same carbon. In the same way alcohols add to aldehydes and ketones to form geminal di alkoxide known as acetal containing two alkoxide groups attached to the same carbon. Acetals derived from ketones are once called as ketals but this term is now obsolete. The process of acetal formation passes through an intermediate called hemi acetal in which the carbonyl carbon has one alkoxide and one hydroxyl group. Acetal formation follows the mechanism of all reversible acid catalysed nucleophilic addition of alkoxide ion on carbonyl carbon. It is a reversible reaction, and it forms acetal when there is excess alcohol and it reverses when there is excess water. Fig 1. Acetal formation www.chemistryscore.com/acetal-formation-rohh/ The acetal formed can be symmetric acetal if the two alkoxide groups are from the same alkyl group or it can be mixed acetal if they are from different alkyl group. Acetal formation acts as a protecting mechanism for protecting the carbonyl group of aldehyde or ketone in syntheses. Certain chemicals like acetaldehyde and formaldehyde are conveniently stored in acetal form Hemiacetals and acetals are important functional groups appear in sugars and carbohydrates. Topic sections: The mechanism of acetal formation: The mechanism of acetal formation involves the following seven steps. The role of the acid catalyst is to make the nucleophilic attack by the alcohol more effective. Step 1 : Protonation of the carbonyl. The carbonyl oxygen is protonated by the presence of acid and thus making the carbonyl carbon more electrophilic so that the nucleophilic attack by the alcohol in step 2 will be more effective. Step 2 : Nucleophilic attack by the first alcohol. Step 3 : Deprotonation to form hemi acetal intermediate. Step 4: Protonation of the alcohol ( OH) of the hemi acetal intermediate Step 5: Removal of water. Step 6: Nucleophilic attack by the second alcohol Step 7: Deprotonation to form acetal. Fig 2 Mechanism of Acetal Formation https://upload.wikimedia.org/wikipedia/commons/e/e1/Acetal_formation.png Carbonyl protection by Acetal Formation: In the acetal formation mechanism the formation of hemiacetal can be catalyzed either by acid or base. However the conversion of hemiacetal to acetal which involves removal of water can only be effected by acid. Since the conversion of hemiacetal to acetal cannot be carried by a base, the reversal of acetal to hemiacetal also will not take place in a base. Thus the acetals are stable in basic or neutral medium. This property is used in protecting the acetal and regenerating the carbonyl in organic syntheses. Consider the conversion of p- bromo acetophenone to p-hydroxymethyl acetophenone. One of the way is to convert p-bromoacetophenone in to Grignard reagent and then to react with formaldehyde. But, the Grignard reagent derived from one molecule of the starting material would react with the carbonyl group of another molecule, and thus the ketone group would not survive this reaction. Fig 3. Carbonyl protection by acetyl formation However, the ketone can be protected by acetal formation with ethylene glycol. The acetal is then reacts with Mg to form the Grignard reagent and the conversion can be conveniently effected. Note that it is all done in basic or neutral medium after the acetal formation. In the last step the ketone is regenerated by acid hydrolysis to the required product. Storage of aldehydes: Acetals can also be formed without the need of any alcohol. Some of these acetal formation finds useful in a convenient way to store the aldehyde. Acetaldehyde, when treated with a trace of acid, readily forms a cyclic acetal called paraldehyde . Each molecule of paraldehyde is formed from three molecules of acetaldehyde. Paraldehyde boils at 125 deg C whereas acetaldehyde boils at 20 deg C and hence acetaldehyde can be stored in the form of paraldehyde Similarly formaldehyde forms the acetal polymer paraformaldehyde, which precipitates from concentrated formaldehyde solution. Paraformaldehyde is a solid and can be conveniently stored and formaldehyde is liberated as a gas on heating paraformaldehyde. Acetal formation in Sugars and carbohydrates: Sugars and carbohydrates are the compounds formed by the glycosidic linkage between monosaccharides. The glycosidic linkage in sugars are nothing but acetal formation. Since the monomers are glucose and the like molecules, the acetal linkage in carbohydrates and sugars are called glycosidic linkage. A glycosidic linkage is the bond that forms when the hemiacetal OH of the anomeric carbon of carbohydrate reacts with another alcohol group to form an acetal. For example lactose is a disaccharide formed by the acetal formation between one galactose molecule with one glucose molecule. Fig 4. Lactose https://www.uoguelph.ca/foodscience/book-page/lactose Here the anomeric carbon of Galactose is in acetal form and the anomeric carbon of glucose is in hemi aetal form. Key Takeaways: Acetal formation is the process of conversion of carbonyl group of an aldehyde or ketone in to a gem di alkoxide or ethers of gem-diols which are open chain , cyclic or polymeric. Acetal formation with alcohol follows all reversible acid catalyzed nucleophilic addition mechanism. Acetal formation can happen with diols or even without alcohols to form cyclic and polymeric acetals. The glycosidic linkage found in sugars and carbohydrates are due to acetal formation. Acetal formation is useful in protecting the carbonyl group in chemical syntheses. Acetaldehyde and formaldehyde can be better stored in acetal form.