Feb 7 SI
advertisement

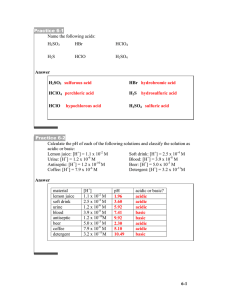
SI Chem 178 Chapter: 15 (Le Chatelier’s,) and 16 Leader: Emily Feb 7th, 2013 Let’s Not Forget… Ch 13: See pg 529 for conversion of molality and molarity… (Separate sheet of paper!) Ch 13: Is the solubility of a gas in a closed chamber affected by pressure? What about solids and liquids? Ch 13: As temperature increases, solubility generally: increases/decreases Which type of solution can we make using this property? Is that solution stable? Colligative Properties depend upon: species dissolved/concentration of species Definition of: Arrhenius Acid/Base? Definition of: Bronsted-Lowry Acid/Base? What’s another word for “basic”? Any questions from Ch 15? … (Kc/Kp – equations, values/magnitude, Q, heterogeneity/homogeneity, ICE, Le Chatelier’s) For the Quizzy… Le Chatelier’s (See book pages: 630-639, awesome diagram on pg 631) Kc is affected by… ? What information is helpful in determining which way a reaction will go with temperature change? Think H values… **REMEMBER YOUR STRONG ACIDS AND BASES!** Conjugate Acid and Base Pairs: List the conjugate species of the following chemicals. Note whether they are an acid or base. What is unique about the starred (*) examples? NH3 H3PO4 HCl Li(OH)2 NaOH *HCO3H2SO4 *HSO4What is the value of Kw? ___________________ What is the equation that relates pH and pOH? _____________________________ What does that “p” from above mean? _______________________ When can we use Kw as our Kc for an equilibrium acid/base reaction? A solution is acidic/basic when its [H+] > 1x10^-7M A solution is acidic/basic when its [H+] < 1x10^-7M A solution is Acidic/basic when its [OH-] > 1x10^-7M A solution is acidic/basic when its [OH-] < 1x10^-7M I dissolved 4.3x10^-6 moles of HI in 1.25L of water. What is my proton concentration, hydroxide ion concentration, pH, and pOH? (What information must you interpret here!?) How would you go from pOH to H+ concentration? Example: I have 0.08M OH- in solution, what is my H+ concentration (hint, use equation mentioned above…)? Weak Acid Calculations: Ka and % Ionization What is the GENERAL equation for Ka? What do all the components mean? Write it out for HCl (please). What are the Ka and equilibrium concentrations of components in a solution of HF at a pH of 2.96? What is the above acid’s % dissociation? (Write out the basic equation, then apply it!) ON A SEPARATE SHEET: I have 0.4M HNO2 (Ka=4.5x10^-4 @ 25 Deg C)… What is my pH?