Properties of Water
advertisement
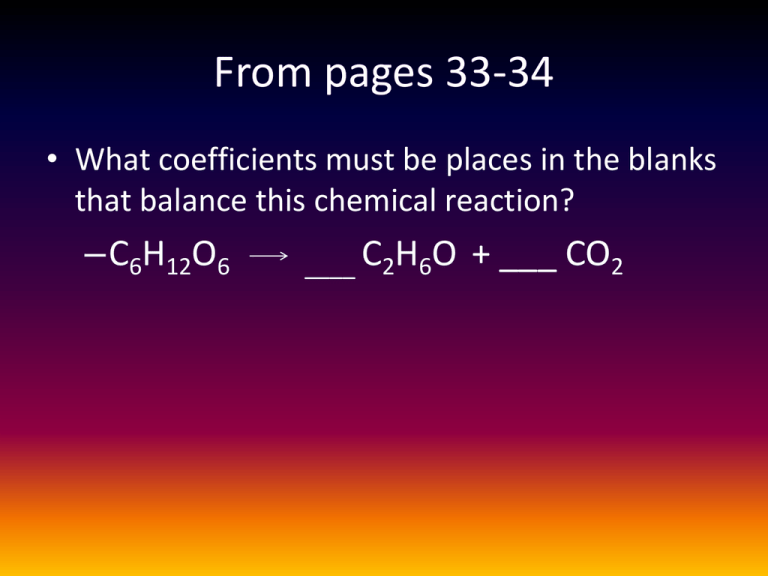
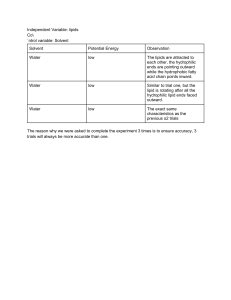
From pages 33-34 • What coefficients must be places in the blanks that balance this chemical reaction? –C6H12O6 ____ C2H6O + ___ CO2 From pages 33-34 • Which of the following statements correctly describes any chemical reaction that has reached equilibrium? – The concentration of products equals the concentration of reactants. – The rate of the forward reaction equals the rate of the reverse reaction. – Both forward and reverse reactions have halted. – The reaction is now irreversible. – No reactants remain. Properties of Water Facts • Cells are about 70-95% water • Most cells are surrounded by water • Water is a main reactant in a lot of chemical reactions of life • Exists in all 3 states of matter – Solid (ice), liquid, vapor Polarity • • • • What is electronegativity? What is nonpolar? What is polar? Draw and explain Figure 2.11 • PAGE 29 (37-top 38) Figure 2.11 Figure 2.11 • Draw and explain Figure 3.1 (Page 38) Figure 3.1 • Polar covalent bond • 2 H bonded to 1 O • Electrons shared unevenly allowing for H bonding between water molecules • H bonds=weak covalent bond Assignment • Once I assign you a group, obtain a computer. Go to our classroom and open the assignment titled “Water properties”. Properties of WAter • Surface Tension • Capillary Action • High specific heat capacity – Ability to Stabilize Temperature – High heat of vaporization/evaporative cooling • Expansion upon freezing • Versatility as a solvent Surface tension • What characteristic leads to the surface tension of water? • What bonds form the invisible film on the surface of water? • Are these bonds only found on the surface? Capillary action • Define capillary action. • What characteristic(s) lead to this property? • Relate this to both straws and plant hydration. Specific heat • What is the “book definition” of specific heat capacity? • HOW is water able to hold on to heat? • Does sweating cool the body? Explain. • How is high specific heat of water beneficial to life on Earth? Expansion upon freezing • What is the movement of molecules in a frozen solid state? • Answer expand or contract: – Water moving above 4 degrees _____ – Water moving from 0 degrees to 4 degrees _____ – Water moving from 4 degrees to 0 degrees _____ – Water moving below 0 degrees _____ • In a crystalline structure of ice, each water molecule has how many “other partners”? Water is the solvent of life • • • • • What is a solution? What is a solvent? What is a solute? What is an aqueous solution? What quality of water enables water to be such a versatile solvent? Figure 3.7 • Draw and explain Figure 3.7 (page 42). Hydrophilic vs Hydrophobic • What is hydrophilic? • What is hydrophobic? • PAGE 42-43