surface tension
advertisement

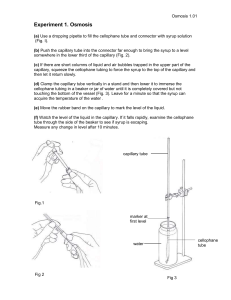
Microfluidics ENGR 1182.03 Pre Lab Review: hydrostatic pressure Pa The pressure at the bottom of an open container filled with liquid: P Pa gh h P Pa atmospheric pressure = density of the liquid g = acceleration due to gravity h = height of liquid Example: for water (r = 1000 kg/m3) at sea level (g = 9.80 m/s2) the hydrostatic pressure at a depth of h =10 m is: P P Pa gh 1000 kg/m3 9.80 m/s 2 10 m kg kg m N P 98,000 98,000 2 2 98,000 2 0.967 atm 2 ms m s m Pressure conversion factors: 1 atm = 1.01325 × 105 N/m2 = 14.696 psi Surface tension concave meniscus When a glass tube is immersed in water, liquid rises inside the tube due to surface tension and a concave meniscus forms. Surface tension can be thought of as a force, acting along the air/water/glass contact line, that “pulls” the liquid up the tube. Surface tension is caused by intermolecular forces. Capillary flow Surface tension can therefore cause fluid to flow in a capillary channel. Important factors are: tube orientation and the gravitational constant (g) diameter of tube density (r) and surface tension (g) of the liquid chemical nature of the tube walls A capillary “valve” If a tube initially filled with water is allowed to slowly drain, not all of the liquid drains out. In addition to surface tension at the top of the liquid, surface tension also acts to counter the expansion of surface area at the exit, and therefore prevents further flow. This is the basic principle behind a capillary check valve; undesired flow can be resisted by introducing a sudden expansion in a flow channel. Let’s take another look at a vertical capillary tube immersed in liquid. Liquid spontaneously rises until it reaches an equilibrium height. P1 The hydrostatic pressure at height hA is: P2 P1 - P2 = rghA P2 = P1 – rghA hA P1 Note that pressure P2 (just beneath the surface) is not equal to P1! This is a consequence of this interface being curved. P1 Now let’s take another look at a capillary tube that is initially filled and allowed to slowly drain until it reaches the equilibrium state shown here. P2 At equilibrium, P3 = P2 + rghB Substituting equation for P2: P3 = P1 – rghA + rghB hB P3 – P1 = rg(hB – hA) P3 P1 (P3– P1) is the pressure rating of this capillary valve. A pressure > (P3– P1) is required to make liquid flow through this valve. Capillary check valves Capillary check valves can be used to prevent undesired flow into or out of a fluid reservoir in a device with micronsized channels. Learning Objectives of Lab Understand capillary flow and how a capillary valve works. Explore how the flow of fluid in a micro-channel depends on pressure and geometry. Practice delivering and cleansing mock samples.