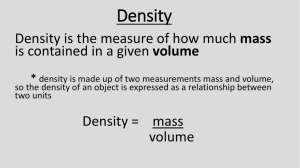Density Notes
advertisement

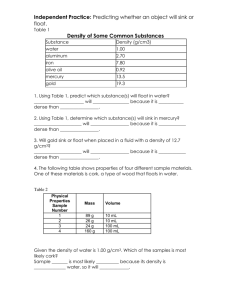
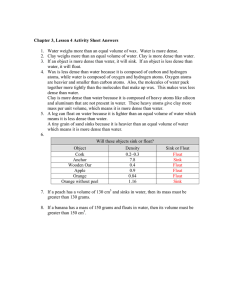
Do Now: 1. Define mass. ___________________________________________________________________________ 2. Define volume. _________________________________________________________________________ 3. What do you think density means? 4. Why do you think some things float in water? Label your Table of Contents: “Density.” Density Notes Density is the mass of a material in a given volume. Density is often written in units of g/cm3 or g/mL. Let’s take “g/cm3” and break it down to see where the units came from. Where did the “g” come from? It came from the unit of measure for mass. Where did the “cm3” come from? It came from the unit of measure for volume. The formula for density is D=M/V which means Density = Mass divided by Volume If I had a cup of juice with a mass of 24 g and a volume of 6 mL, what is its density? Write out how you found the density. 24g/6mL = 4g/mL The density was found by taking the mass (24g) divided by the volume (6mL). What is the density of a smoothie with a mass of 30 g and a volume of 10mL? Write out how you found the density. 30g/10mL = 3g/mL The density was found by taking the mass (30g) divided by the volume (10mL). What is the density of a pickle with a mass of 20g and a volume of 4cm3? Write out how you found the density. 20g/4mL = 5g/cm3. The density was found by taking the mass (20g) divided by the volume (4cm3). What is the density of a block of Styrofoam with a mass of 2g and a volume of 8cm3? Write out how you found the density. 2g/8cm3 = .5g/cm3 The density was found by taking the mass (2g) divided by the volume (8cm3). If these boxes have the same volume, which has a higher density? Circle the box with the higher density. To the right of the boxes, write about how you knew which one had the higher density. Water has a density of 1 g/mL Anything less dense than 1 g/mL will float in water Anything more dense than 1 g/mL will sink in water A goobleton (made up word, don’t worry about it) has a density of 1.4 g/mL. Will it sink or float in water? How do you know? Answer: It will sink because it is more dense than the density of water (1 g/mL). 1.4>1 A harkyparky (once again, don’t worry about it) has a density of 0.98 g/mL. Will it sink or float in water? How do you know? Answer: It will float because it is less dense than the density of water (1g/mL). 0.98<1