The Mole - astchemistry
advertisement

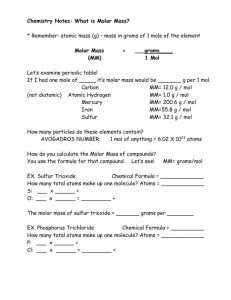
Unit 8 The Mole 23 6.02 X 10 The Mole A counting unit Similar to a dozen, except instead of 12, it’s 602 billion trillion 602,000,000,000,000,000,000,000 Avogadro’s number= 6.02 X 1023 (in scientific notation) This number is named in honor of Amedeo Avogadro (1776 – 1856), who studied quantities of gases and discovered that no matter what the gas was, there were the same number of molecules present Just How Big is a Mole? Enough soft drink cans to cover the surface of the earth to a depth of over 200 miles. If you had Avogadro's number of unpopped popcorn kernels, and spread them across the United States of America, the country would be covered in popcorn to a depth of over 9 miles. If we were able to count atoms at the rate of 10 million per second, it would take about 2 billion years to count the atoms in one mole. One mole of $100 bills stacked one on top of another would reach from the Sun to Pluto and back 7.5 million times. One mole of marbles would cover the entire Earth (oceans included) for a depth of three miles. Homework How long will it take to spend a mole of Lps.1.00 if they were being spent at a rate of a billion per second? The Mole 1 dozen cookies = 12 cookies 1 mole of cookies = 6.02 X 1023 cookies 1 dozen cars = 12 cars 1 mole of cars = 6.02 X 1023 cars 1 dozen Al atoms = 12 Al atoms 1 mole of Al atoms = 6.02 X 1023 atoms Note that the NUMBER is always the same, but the MASS is very different! Mole is abbreviated mol (big difference!!) A Mole of Particles Contains 6.02 x 1023 particles 1 mole C = 6.02 x 1023 C atoms 1 mole H2O = 6.02 x 1023 H2O molecules 1 mole NaCl = 6.02 x 1023 NaCl formula units (ionic compounds are not molecules so they are called formula units) 6.02 x 1023 Na+ ions and 6.02 x 1023 Cl– ions Avogadro’s Number as Conversion Factor 6.02 x 1023 particles 1 mole or 1 mole 6.02 x 1023 particles Note that a particle could be an atom, ion, formula unit OR a molecule! Ex. 1 How many atoms are in 0.67mol of Al? = 0.67 mol Al 6.02x 1023 atoms Al 1 mol Al = 4.0 x 1023 atoms Al Remember to round answer to correct number of significant digits and don’t forget to write units Ex. 2 A sample of gold contains 5.21 x1023 atoms of gold, how many moles is this? = 5.21x 1023 atoms Au 1 mol Au 6.02x 1023 atoms Au = 0.865 mol Au Remember to round answer to correct number of significant digits and don’t forget to write units Learning Check 1. Number of atoms in 0.500 mole of Ag a) 500 Ag atoms b) 6.02 x 1023 Ag atoms c) 3.01 x 1023 Ag atoms 2.Number of moles of S in 1.8 x 1024 S atoms a) 1.0 mole S atoms b) 3.0 mole S atoms c) 1.1 x 1048 mole S atoms Learning Check 1. Number of atoms in 0.500 mole of Ag a) 500 Ag atoms b) 6.02 x 1023 Ag atoms c) 3.01 x 1023 Ag atoms 2.Number of moles of S in 1.8 x 1024 S atoms a) 1.0 mole S atoms b) 3.0 mole S atoms c) 1.1 x 1048 mole S atoms Molar Mass The Mass of 1 mole (in grams) Equal to the numerical value of the average atomic mass (get from periodic table) 1 mole of C atoms = 12.0 g 1 mole of Mg atoms = 24.3 g 1 mole of Cu atoms = 63.5 g Units: grams/mole or g/mol Other Names Related to Molar Mass Molecular Mass/Molecular Weight Formula Mass/Formula Weight Learning Check! Find the molar mass (usually we round to the tenths place) A. 1 mole of Br atoms = B. 1 mole of Sn atoms = 79.9 g/mole 118.7 g/mole Molar Mass of Molecules and Compounds The molar mass of a compound is equal to the sum of the atomic masses of all the atoms in the compound. 1 mole of CaCl2 = 111.1 g/mol 1 mole Ca x 40.1 g/mol = 40.1 g/mol + 2 moles Cl x 35.5 g/mol = 71.0 g/mol = 111.1 g/mol CaCl2 Molar mass for calcium nitrate, Ca(NO3)2 Ca : N: O: 1 atom x 40.1 g/mol = 40.1 g/mol 2 atoms x 14.0 g/mol = 28.0 g/mol 6 atoms x 16.00 g/mol = 96.0 g/mol + 164.1 g/mol Learning Check! A. Molar Mass of K2O = ? Grams/mole B. Molar Mass of antacid Al(OH)3 = ? Grams/mole Learning Check! A. Molar Mass of K2O = ? Grams/mole K : 2 x 39.1 = 78.2 O : 1 x 16.0 = 16.0 + 94.2 g/ mol B. Molar Mass of antacid Al(OH)3 = ? Grams/mole Al: 1 x 27.0 = 27.0 O: 3 x 16.0 = 48.0 H: 3 x 1.00 = 3.0 + 78.0 g/mol Learning Check Prozac, C17H18F3NO, is a widely used antidepressant that inhibits the uptake of serotonin by the brain. Find its molar mass. (answer: 304 g/mol) Calculations with Molar Mass molar mass Grams Moles To convert between grams and moles, use the molar mass of the substance as a conversion factor. Converting Moles to Grams Aluminum is often used for the structure of light-weight bicycle frames. How many grams of Al are in 3.00 moles of Al? 3.00 moles Al ? g Al To convert between grams and moles, Calculate the molar mass of the substance 1. Molar mass of Al 1 mole Al = 27.0 g Al 2. Conversion factors for Al 27.0g Al 1 mol Al or 1 mol Al 27.0 g Al 3. Setup 3.00 moles Al Answer x 27.0 g Al 1 mole Al = 81.0 g Al Converting grams to moles Acetylsalicylic acid is the chemical name for the substance we commonly know as aspirin, C6H4(OCOCH3)CO2H. How many moles of aspirin are 255g of aspirin? 255 g aspirin ? Mol aspirin 1. Molar mass of aspirin 1 mole = 180.0 g aspirin 2. Conversion factors for aspirin 180.0g 1 mol or 1 mol 180.0 g 3. Setup 255 g aspirin Answer x 1 mole aspirin 180 g aspirin = 1.42 mol aspirin Learning Check! The artificial sweetener aspartame (NutraSweet) formula C14H18N2O5 is used to sweeten diet foods, coffee and soft drinks. How many moles of aspartame are present in 225 g of aspartame? Answer: 0.765 mol aspartame Learning Check A recipe requires 0.629 mol of sucralose, SPLENDA (C12H19Cl3O8). How many grams is this? Answer: 250. g splenda Conversions: particles Grams 6.02 X 1023 particles = 1 mole AND 1 mole = molar mass (grams) We can convert between particles and grams (Two step process) You can’t go directly from atoms to grams!!!! You MUST go thru MOLES. Calculations molar mass Grams Avogadro’s number Moles particles Everything must go through Moles!!! Atoms/Molecules and Grams How many atoms of Cu are present in 35.4 g of Cu? Plan: g Cu mol Cu atoms Cu Step 1: converting g Cu to mol Cu using molar mass 35.4 g Cu 1 mol Cu = 0.5566 mol Cu 63.6 g Cu Step 2: converting mol Cu to atoms Cu using Avogadro’s number. 0.5566 mol Cu 6.02 x 1023 atoms Cu= 3.35x1023 atoms Cu 1 mol Cu Atoms/Molecules and Grams How many atoms of Cu are present in 35.4 g of Cu? Plan: g Cu mol Cu atoms Cu Combining step 1 and 2 35.4 g Cu 1 mol Cu 6.02 x 1023 atoms Cu= 3.35x1023 atoms Cu 63.6 g Cu 1 mol Cu Atoms/Molecules and Grams What is the mass of 5.54x1024 molecules of CO? Plan: molecules CO mol CO g CO Step 1: converting molecules CO to mol CO using Avogadro’s number. 5.54x1024 molecules of CO 1 mol CO = 9.2026 mol CO 6.02x1023 molecules CO Step 2: converting mol CO to g CO using molar mass 9.2026 mol CO 28.0 g CO = 258g CO 1 mol CO Learning Check! How many atoms of K are present in 78.4 g of K? (answer: 1.21 x 1024 atoms K) Learning Check! What is the mass (in grams) of 1.20 X 1024 molecules of glucose (C6H12O6)? (answer:359 g glucose)