Electron Configuration
advertisement

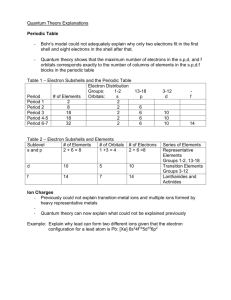
The Elements CH. 2 atomic models electronic configuration oxidation numbers The Models of the Atom Line Spectra What is causing the light to appear when the atom is excited by electricity? Why does the line spectrum of hydrogen have very distinct lines of color and not the complete rainbow, which includes all colors? The Atomic Energy and the Bohr Model of the Atom h The Quantum Theory • The main idea that comes from the Quantum Mechanical Theory and the Schrodinger Equation is Quantum numbers that define the surfaces of the electron densities in which the electrons reside. • There are 3 main quantum numbers, n, l and ml, and a fourth quantum number, ms Principle quantum number, n Schrödinger’s equation provides a number of possible functions that define the shape of the electron cloud about the nucleus, these shapes are the orbitals. The functions can be arranged in sets and subsets. Orbitals are classified in three ways: An electron shell is a collection of orbitals, identified by an integer, n, which ranges in value infinitely up from 1. Electrons with the same value are in the same shell. In chemistry, important shells have n values between 1 and 7. Angular Momentum, l Subshells are groups of orbitals within an electron shell. They are identified by the letters s, p, d, f and through the alphabet beginning with g. In chemical systems, only s, p, d, and f subshells are important. The number of subshells within an electron shell is equal to the n value of the shell. Magnetic, ml Individual orbitals are identified by their direction in space using Cartesian coordinates. The number of orbitals within a subshell depends on the subshell type: s subshells have a single orbital, p subshells have 3 orbitals, d subshells 5 and f subshells 7 orbitals. Values of n, l and ml Example n value l value n = 1, 2,… l = 0, 1, .. n-1 n=1 l=0 n =2 l=0 l=1 n =3 l=0 l=1 l=2 ml value ml = 0, 1, …l ml = 0 ml = 0 ml = +1, 0, -1 ml = 0 ml = +1, 0, -1 ml = +2,+1,0,-1,-2 The orbital shape and l n value l value n=1 l=0 n =2 l=0 l=1 n =3 l=0 l=1 l=2 n =4 l=0 l=1 l=2 l=3 Orbital designation 1s 2s 2px, 2py, 2pz 3s 3p 2 2 2 3dxy, 3dz , 3dyz, 3dxz, 3dx -y 4s 4p 4d 4f The Energy Levels of the orbitals In hydrogen the energy of the orbitals and the order in which they fill are dependant only upon their principle quantum number; however, in atoms with more electrons the order changes. Energy levels in atoms of more than one electron. Notice the shifting of the energy levels, as n increases, energy increases, as l increases, energy increases, but mixing of the energy levels still occur. Orbitals 10s 9s 9p 9d 9f 9g 9h 9i 9j 8s 8p 8d 8f 8g 8h 8i 8j 7s 7p 7d 7f 7g 7h 7i 6s 6p 6d 6f 6g 6h 5s 5p 5d 5f 5g 4s 4p 4d 4f 3s 3p 3d 2s 2p 1s 9k Increasing Energy • Electrons fill different orbitals in a subshell until the subshell is half - filled. (one in each orbital before pairing begins) • If an atom has a filled valence shell, the atom is more stable. • Each electron or the space it occupies can be defined by its four quantum numbers (n, l, ml, ms) • No two electrons can occupy exactly the same space or have the same four quantum numbers. • Some electron configurations defy expectations created by the periodic table, particularly for heavier elements. Periodic Blocks Why do we have those rows at the bottom? H He Li Be B Na Mg Al Si P K Ca Sc Ti Rb Sr Y C N O F Ne S Cl Ar V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr This arrangement takes too much space and is hard to read. Hund’s Rule A full energy level is very stable, of lowest energy, a half filled shell is almost as stable as a fully filled shell and a partially filled shell is the least stable of highest energy. The orbitals will go from partially filled to half filled by putting one electron in each orbital before pairing the spins. Electron Configurations • Complete electron configurations • noble gas electron configurations showing the noble gas and the remaining valence electrons • valence electron configuration Fe 4s 3d +2 Fe 4s 3d +3 Fe 4s 3d Rules for assigning oxidation numbers The sum of the oxidation number of all atoms must equal the net charge of the species. In compounds: Group IA are +1. Group IIA are +2. B and Al are +3, and F is -1. Hydrogen is +1 except when combined with a metal. Then it is -1. Oxygen is -2 except for peroxides and superoxides. Elements in their elemental state have an oxidation number of zero. Li Be B +1 +2 +3 H He C N +4 +5 +4 -2 +3 +2 -4 +1 -3 O F -1 -2 -1 S Cl Ne +1 Na Mg Al +1 +3 +2 K Ca Sc +1 +2 Ti V 3+ Rb Sr Y +1 +3 Mo Tc Ru Rh Pd Nb Zr +6 +7 +8 +6 +4 +4 Ag Cd +2 +4 Cs Ba Lu Hf Ta +1 +4 +5 +2 +3 Fr Ra Lr +1 +2 +3 +4 -4 P +5 +6 +4 +7 +5 +3 +2 +3 +1 -3 -2 -1 Cr Mn Fe Co As Se Br Cu Ge Ni Zn Ga +6 +7 +6 5+ 6+ +5 +4 +5 +4 +3 +3 +3 +3 +4 +3 +2 +2 +2 +2 +2 +5 +4 Si +4 +3 +6 +4 +3 +2 +2 +2 +1 In +4 -4 3+ 3- +3 +4 +2 +5 +3 -3 +2 W Re Os Ir Pt Au Hg Tl Pb Bi +6 +4 +4 +3 +4 +2 +3 +1 +4 +2 +5 +3 +3 +1 +2 +1 4+ 2- +1 -1 Sn Sb Te +3 +2 +8 +6 +2 +3 +4 +3 +7 +6 +4 +1 +2 I Ar Kr +4 +2 Xe +6 +7 +5 +6 +4 +1 +4 -2 -1 +2 Po +2 At Rn Common oxidation numbers -1 Oxidation numbers and the periodic table Some observed trends in compounds. Metals have positive oxidation numbers. Transition metals typically have more than one oxidation number. Nonmetals and semimetals have both positive and negative oxidation numbers. No element exists in a compound with an oxidation number greater than +8. The most negative oxidation numbers equals 8 - the group number Electron Configuration Activities • Simple Atomic Structure • Atomic Structure - Electron Configuration • Structured Curriculum Lesson Plan





![6) cobalt [Ar] 4s 2 3d 7](http://s2.studylib.net/store/data/009918562_1-1950b3428f2f6bf78209e86f923b4abf-300x300.png)