File - SHAKRA
advertisement
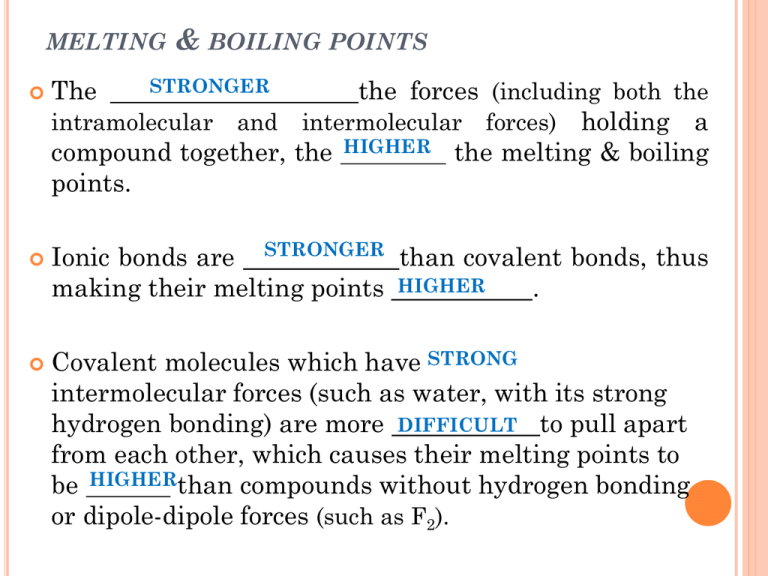
MELTING The & BOILING POINTS STRONGER the forces (including both the intramolecular and intermolecular forces) holding a compound together, the HIGHER the melting & boiling points. Ionic bonds are STRONGER than covalent bonds, thus making their melting points HIGHER . Covalent molecules which have STRONG intermolecular forces (such as water, with its strong hydrogen bonding) are more DIFFICULT to pull apart from each other, which causes their melting points to be HIGHERthan compounds without hydrogen bonding or dipole-dipole forces (such as F2). MELTING &BOILING POINTS OF HALOGENS in covalent molecules which experience only London Dispersion Forces, recall that LARGER molecules have STRONGER London Dispersion Forces; Consider the halogens: Melting Halogen Point (C) Boiling Point (C) F2 -220 -188 Cl2 -101 -34 Br2 -7 58 I2 114 183 VOLATILITY Volatility: is the tendency of a liquid at room temperature to evaporate into a gas In order to evaporate, the liquid molecules must possess enough K.E. to overcome the forces holding the molecules together. Among the halogens, volatility decreases as you go down the group, for exactly the same reasons greater that melting & boiling points increase: mass (and thus greater # of e-) stronger van der Waal forces. CONDUCTIVITY Recall that metallic solids have delocalized valence electrons, which enables them to readily conduct electricity. By contrast, most covalent and ionic solids are nonconductors, because their valence electrons are not delocalized. In covalent molecules, the electrons are shared between just a few atoms. In ionic compounds, the valence electrons are completely lost or gained to other atoms, to satisfy each atom’s octet rule. Ionic substances become conductive in the liquid state, however, because the ions can move (in an ionic liquid, the ions carry the electric current). Likewise, many ionic solutions will conduct electricity, if ionic solid can be dissolved in water, producing an aqueous solution SOLUBILITY Recall that “ like dissolves like ”. In other words, solvents tend to dissolve solutes with similar properties . Polar solvents tend to dissolve polar solutes. ex #1: NH3 dissolves in water to make household cleaners Ammonia & water are both polar, having unshared e–s on the central atom of their molecules. Non-polar solvents tend to dissolve non-polar solutes. ex: I2(s) dissolves in CCl4(l) EX # 2: WATER DISSOLVES SODIUM CHLORIDE BECAUSE The NaCl ions are attracted to the very polar H2O molecules, which play “tug-of-war” with the ionic bonds holding the Na+ and Cl– ions The NaCl dissolves in water because the pull of hundreds of H2O molecules is stronger than the ionic forces holding Na+ and Cl– ions together. Consider also the solubilities of the following alcohols in water: Alcohol Solubility in Water (mol / 100g H2O) name alcohol solubility methanol CH3OH infinite ethanol C2H5OH Infinite propanol C3H7OH Infinite butanol C4H9OH 0.11 pentanol C5H11OH 0.03 hexanol C6H13OH 0.0058 heptanol C7H15OH 0.0008 Alcohols dissolve in water as the – OH group is able to hydrogen bond with H2O molecules, and be pulled in solution Thus, as the length of the alcohol molecule increases, water molecules have less of a chance of hydrogen bonding with the water (more of the interactions with water occur at the non-polar C – C and C – H groups, rather than the polar –OH group). This is why larger alcohol molecules do not dissolve as readily in water.





