Biology Notes 2-2 Properties of Water
advertisement

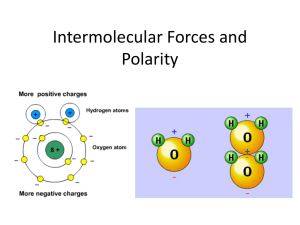
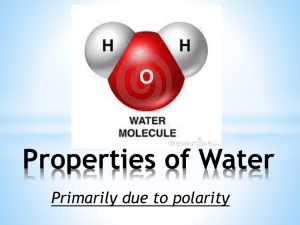
PROPERTIES OF WATER MAIN IDEAS 1. POLARITY 2. HYDROGEN BONDS 3. SOLUTIONS 4. pH SCALE IaN pg. 31 Objective Define & Describe polarity & solutions that are acidic &/or basic using notes & collaboration. I am not a product of my circumstances. I am a product of my decisions. Stephen Covey The Water Molecule • H 2O • Water is a polar molecule –Unequal sharing of electrons –It has a positive and a negative end. –Overall neutral charge (+) (+) (-) (-) Polar Molecules • Electrons are pulled closer to the atom with a larger nucleus in the molecule. Examples of POLAR & NON-POLAR Hydrogen Bonds • Attraction of partial opposite charges between hydrogen and oxygen [for example] • Polar molecules attract other polar molecules . Water’s polarity allows it to dissolve sodium chloride [NaCl] and many other ionic and polar compounds. Cohesion • Attraction between molecules of the same substance. –Ex. Water beading up on smooth surface. –Try floating a metal pin on water. Adhesion • Attraction between molecules of different substances. • Capillary action - a liquid rising due to cohesive & adhesive properties against the force of gravity. Water • Water expands when it freezes. So ice is less dense than liquid water and it floats. Water Properties IaN pg. 30 • Draw a at least 5 water molecules – Color code the molecules by the type of atoms – Illustrate the molecular polarity using (+) (-) – Illustrate & Label the Hydrogen Bonds between the molecules using a ----------• Water Acrostic – 5 sentences about water’s properties. W A T E R