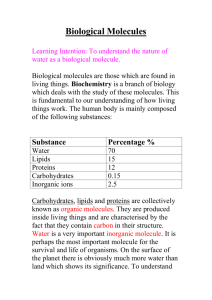
Properties of Water HW
advertisement

Name __________________________ Properties of Water Homework Use the word bank below to answer the following questions. Word Bank: adhesion cohesion covalent dissolve polar positively surface tension universal solvent negatively 1. The hydrogen and oxygen atoms in a water molecule are held together by __________________ bonds. 2. The electrons are not shared equally between the hydrogen atoms and oxygen atom in a water molecule, making it a ___________________ molecule. 3. The polarity of water allows it to ____________________ most substances. Due to this fact, it is often referred to as the ________________________________. 4. Water molecules stick to other water molecules. This property is called ________________________. 5. Hydrogen bonds form between adjacent water molecules because the _________________ charged hydrogen end of one water molecule attracts the ___________________ charged oxygen end of another water molecule. 6. Water molecules stick to other molecules due to its polar nature. This property is called _____________________. 7. _____________________________ is the skin-like surface formed due to the polar nature of water. Refer to the properties of water to answer the following questions: 1. Explain why ice is able to float on water. 2. A bottle of salad dressing contains vinegar (water-based) and oil. You shake the bottle vigorously and then set it on the table. You notice the dressing is evenly mixed. Eventually, the dressing separates into layers of oil over vinegar. Explain why this happens. 3. If you touch the edge of a paper towel to a drop of colored water, the water will move up into (or be absorbed by) the paper towel. Explain why this happens.