INTERMOLECULAR FORCES
advertisement
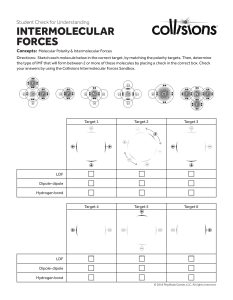
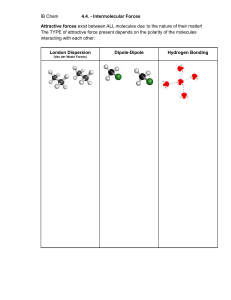
INTERMOLECULAR FORCES Intermolecular Forces • Forces of attraction and repulsion between molecules. • They are weak – if we say that a covalent bond has a strength of 100 then intermolecular forces would range from 0.001 to 15. Dipole-Dipole …A relatively strong force of attraction between two polar molecules. †Can also have ion-dipole forces between ions and a polar molecule Hydrogen Bonding • …Is an exceptionally strong dipole-dipole force • One of the three most electronegative elements, F, O or N must be covalently bonded to a hydrogen (such as HF, H2O, NH3 , CH3OH and CH3NH2). London Forces • The only attractive force nonpolar molecules. • …Basically the electrons in one atom are slightly attracted to the nuclei (positive core) of another atom. • …The greater the number of electrons the stronger the L.F.