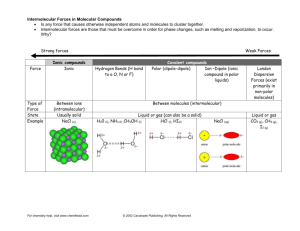
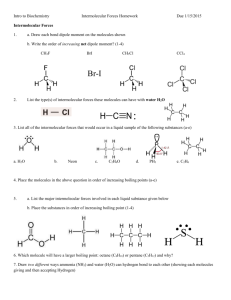
Intermolecular Forces Worksheet
advertisement

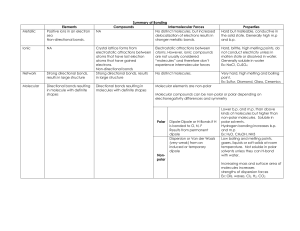
Name_______________________________________________________ Date_________________Period__________ Intermolecular Forces Worksheet Chemistry; Coleman Part One: For each of the following compounds, determine the main intermolecular force they would experience with other molecules of the same substance. SHOW YOUR WORK! A good way to do this is to: Draw the Lewis structure. Determine polarity of the molecule. o If it's polar, does it form hydrogen bonds or just regular dipole-dipole bonds with other molecules of the same substance? o If it's non-polar, how will it be attracted to other molecules of the same substance? 1) N2 ________________________________ 2) sulfur dioxide ________________________________ 3) NH3 ________________________________ 4) SiH2O _________________________________ 5) Fluorine monocarbon mononitride _________________________________ Part Two: For each of the following pairs of compounds, determine whether the molecules are polar or not based on their Lewis Dot Structures, shape, and polarity of their bonds. Then determine what type of intermolecular forces would attract the two molecules to each other. Show all your work. 1) CHCl3 and H2O 2) CF2H2 and H2O 3) SO2 and NH3