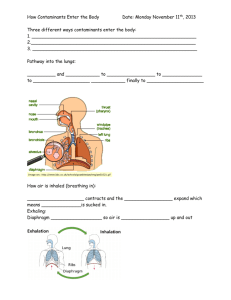
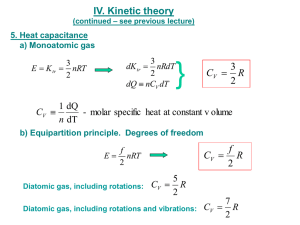
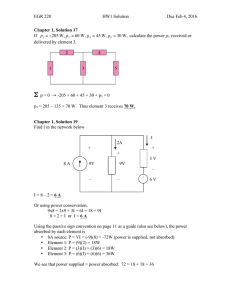
Specific heat capacity
advertisement

Today’s lesson • Define specific heat capacity • Solve problems involving specific heat capacities Specific heat capacity Specific heat capacity is the amount of energy needed to raise the temperature of unit mass of a substance by 1°C. So if a substance has a higher specific capacity, then it takes more energy to raise or lower its temperature. Specific heat capacity of water = 4.186 J/g.°C Specific heat capacity of kerosene = 2.010 J/g.°C Specific heat capacity of mercury = 1.40 J/g.°C Calculations using S.H.C. Energy absorbed = Mass x Specific Heat capacity x Temp rise J g J.g-1.C-1 Q = mcΔT C For example 500 g of olive oil is heated until its temperature rises by 120 C. If the specific heat capacity of olive oil is 1.97 J.g-1.C-1, how much heat energy was used? Energy absorbed = Mass x Specific Heat capacity x Temp rise Energy absorbed = 500 x 1.97 x 120 Energy absorbed = 118200 J Things to note: • If specific heat capacity is constant, the temperature will rise at a uniform rate so long as the power input is constant and no energy is lost to the outside. • There are large potential heat losses if the substance is not well insulated. These can be accounted for in some experiments (how?). • You should be able to think of a number of reasons why your value does not match that in the data book.