Chapter 16.1 - Brookwood High School
advertisement

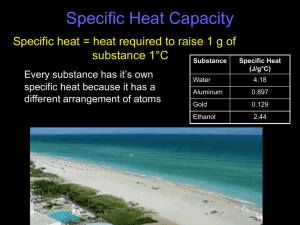
Chapter 16.1 Specific Heat Specific Heat The specific heat of a substance is the heat required to raise the temperature of one gram of that substance one degree centigrade. Every substance has it’s own unique specific heat because every substance is composed of different combinations of atoms. The temperature of matter is a direct measure of the motion of the molecules; the greater the motion, the higher the temperature: Motion requires energy: the more energy matter has the higher the temp. Typically this energy is supplied by heat. Heat loss or gain by matter is equivalent energy loss or gain. Specific Heat Water has a very high specific heat and it does not change temperature quickly. Therefore, land masses that are close to large bodies of water have a very moderate climate. Common Specific Heats Heat Absorbed and Released Energy gained or lost by a substance is directly related to: 1. the mass 2. the change in temperature of that substance Formula to calculate the values: q = c x m x ∆T q = heat released or gained C = specific heat m = mass ∆ = change in temperature Specific Heat Practice Problems 1. A serving of Chubby Hubby Ice Cream contains 2675 kJ of energy. If you ate a serving of this ice cream every night for four weeks and didn’t change your diet and exercise routine, how much weight would you gain in four weeks? ( One lb = 4000 Cal) 2. If the temperature of 50 g of ethanol increases from 21° C to 78.8° C, how much heat has been absorbed by ethanol? (Specific heat for ethanol = 2.44 J/g°C) Specific Heat Practice Problems 3. If the temperature of a 500 g piece of aluminum increases from 20° C to 200° C, how much heat has been absorbed by the aluminum? The specific heat of aluminum is 0.897 J/g°C. 4. A 10.0 g nugget of pure gold absorbed 550 J of heat. What was the final temperature of the gold if the initial temperature was 21°C. The specific heat of gold is 0.129 J/g°C.