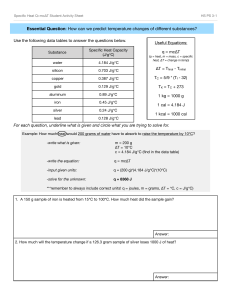
Specific heat
advertisement

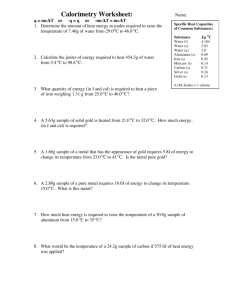
Things they should know Conduction Convection Radiation Temperature – average KE. Faster moving = more KE = higher temp What we will talk about Specific heat - handle on a pot…is it metal? - put a pot on the stove with water….which gets hotter faster? o heat capacity = amount of energy needed to raise 1°C - “insulators” high heat capacity – not much temp change - What other factors change temp change… o bathtub vs coffee cup mass o metal vs. water specific heat - equation o Q = mcΔt Q = heat m = mass C = specific heat Δt = tf – ti - Used to find how hot things will get so they don’t break down o Ex: Enzymes in body o Ex: Water’s high heat capacity…good news for us o Ex: radiator on a vehicle to cool engine. water can absorb a lot of energy o Nuclear Plants water cools down reaction. A 5.10 kg cast iron skillet is heated on a stove from 295 K – 450 K. How much heat is being transferred to the iron? Q = mCΔT You will need the table in your book for this one… What is the temperature of each of the following substances if 8500 J of energy is added to each one? A) water B) Aluminum C) Silver A 2350 kg granite tombstone absorbs 2.8 x 107 J of energy form the sun to change its temperature from 5.0°C to 20.0°C. Determine the specific heat of granite.