02 – Energy Changes in one substance
advertisement
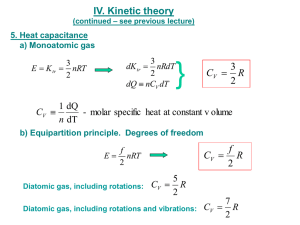
Unit 07 - Thermodynamics Calculating Energy Changes in One Substance: When energy is given off in the form of heat, this value can be measured or calculated Recall that the base unit for energy is the joule (J) If the amount of heat given off by a substance is to be measured, its specific heat capacity must also be known. Specific heat capacity is defined as the amount of heat required to raise 1 g of a substance 1˚C. Therefore the units for a substance’s specific heat capacity is J/g˚C. The specific heats of several substances will be provided for you (Page 42 in your text has a few) Calculating Energy Changes: There is a WAY COOL FORMULA we can use to calculate the amount of energy absorbed or released from a substance when the temperature changes. It is: Q = mc∆t Q = energy (J) absorbed or released m = mass of the substance (g) c = specific heat capacity (J/g°C) ∆t = change in temperature (°C) tfinal - tinitial Calculating Energy Changes: Please note: When using the Q = mc∆t formula: ○ If heat is absorbed, Q will be positive. ○ If heat is released, Q will be negative. The value of Q itself will always be positive. You need to understand mentally if Q is representing heat being absorbed or heat energy being released; the negative sign is simply representing if it’s absorbed or released Example 1: What quantity of heat is required to raise the temperature of 100.g of liquid water 10.°C? Example 2: What quantity of heat is released when 420.0g of lead cools from 85°C to 25°C? The specific heat capacity of lead is 0.13J/g°C.