9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Naming Binary Molecular
Compounds
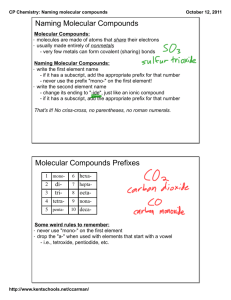
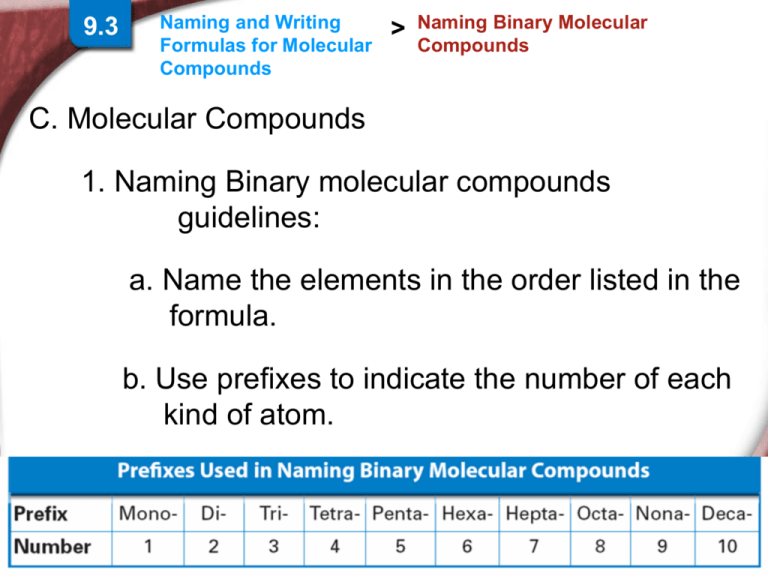
C. Molecular Compounds
1. Naming Binary molecular compounds
guidelines:
a. Name the elements in the order listed in the
formula.
b. Use prefixes to indicate the number of each
kind of atom.
Slide
1 of 15
© Copyright Pearson Prentice Hall
9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Naming Binary Molecular
Compounds
i. Omit the prefix mono- if formula
contains only one atom of the first
element in the name.
ii. The suffix of the name of the second
element is -ide.
2. Writing Formulas: prefixes in the name to tell you
the subscript of each.
a. Guidelines:
Slide
2 of 15
© Copyright Pearson Prentice Hall
9.5
i. An -ide ending indicates a binary
compound.
ii. An -ite or -ate ending means a
polyatomic ion that includes oxygen is
in the formula.
iii. Prefixes indicates that the compound
is molecular.
iv. Roman numeral shows the ionic
charge of the cation.
b. Examples: Silicon carbide > SiC
Potasium sulfate > K2SO4
Fe(III) hydroxide > Fe(HO)3
© Copyright Pearson Prentice Hall
Slide
3 of 15
Naming
Compounds
Given
a
Formula
END OF SHOW
Writing a
Compound’s
Formula
Given a Name
END OF SHOW
CFU
1. The law of definite proportions states that
in samples of any chemical compound,
the elements are always in the same
proportion by
a. mass.
b. volume.
c. group number.
d. period number.
Slide
6 of 15
© Copyright Pearson Prentice Hall
CFU
2. You want to write the chemical formula for
iron(II) chloride. Based on Figure 9.22, after
identifying symbols, what is the correct next
step in the flowchart?
a. Group A elements
b. Roman numerals
c. Balance charges
d. Polyatomic ions
Slide
7 of 15
© Copyright Pearson Prentice Hall
CFU
3. Using the flowchart in Figure 9.20, if you
determine that the name of an ion ends in -ite
or -ate, the ion is a
a. polyatomic cation.
b. polyatomic anion.
c. transition metal cation.
d. group A anion.
Slide
8 of 15
© Copyright Pearson Prentice Hall
CFU
4. Which of the following compounds is named
INCORRECTLY?
a. CS2, carbon disulfide
b. BCl3, boron trichloride
c. IF7, iodine heptafluoride
d. PCl5, phosphorus hexachloride
Slide
9 of 15
© Copyright Pearson Prentice Hall
CFU
5. Which of the following molecular compounds
is named INCORRECTLY?
a. SbCl3, antimony trichloride
b. C2O5, dicarbon pentoxide
c. CF4, carbon tetrafluoride
d. H3As, hydrogen arsenide
Slide
10 of 15
© Copyright Pearson Prentice Hall
CFU
6. The correct formula for tetraphosphorus
trisulfide is
a. P3S4
b. S3P4
c. P4S3
d. S4P3
Slide
11 of 15
© Copyright Pearson Prentice Hall
Proficiency Practice
181. Organic compounds are molecular compounds that
contain carbon (C). They often have hydrogen (H),
nitrogen (N), sulfur (S) and phosphorus (P).
Identify the formula for organic compound Methane.
a. (NH3)
b. (PO4)
c. (CH4)
d. (SO2)
Slide
12 of 15
© Copyright Pearson Prentice Hall
Proficiency Practice
182. The compound water is represented by the formula
H2O. Water is the only compound with this formula
because
a. only water contains atoms of the elements hydrogen
and oxygen.
b. atoms of hydrogen and oxygen are identical to each
other in a reaction.
c. atoms can never be created or destroyed by physical or
chemical reactions.
d. a specific compound always has the same relative
numbers and kinds of atoms.
© Copyright Pearson Prentice Hall
Slide
13 of 15