2.7 Molecular Compounds: Chemical Formulas and Naming
advertisement

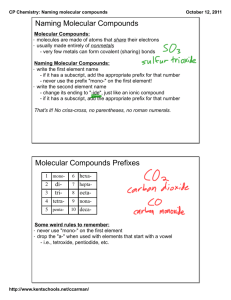
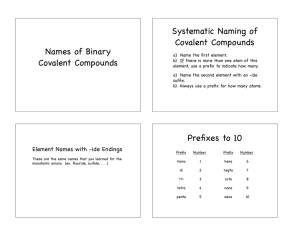
2.7 Molecular Compounds: Chemical Formulas and Naming (Section 7.4 pg 192-196) • Molecular compounds form covalent bonds, so we don’t pay as much attention to ion charges – there are different rules for naming & writing them. • We are going to use a lot of prefixes: • Don’t worry about memorizing these – they’re in the Data Pages!!! • To write the name of a molecular compound from the formula, follow these steps: – Make sure both elements are non-metals. – Write the name of the first element with the correct prefix (please note – if there is only ONE atom of the first element, do not use ‘mono’ e.g. CO2 = carbon dioxide) – Write the name of the second element with the correct prefix. – Change the ending of the second element to -ide. – For example, C4S7 = tetracarbon heptasulfide • To write the formula from the name, you simply write down the symbol for each element and then write an appropriate subscript for the number of the prefix for each element. • For example, triphosphorus octafluoride = P3F8