Naming Molecular Compounds Molecular Compounds Prefixes
advertisement

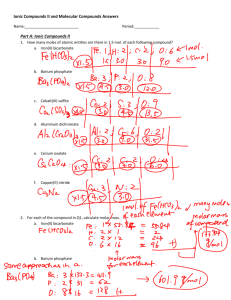
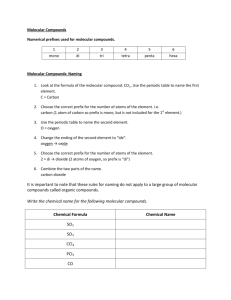
CP Chemistry: Naming molecular compounds Naming Molecular Compounds Molecular Compounds: · molecules are made of atoms that share their electrons · usually made entirely of nonmetals - very few metals can form covalent (sharing) bonds Naming Molecular Compounds: · write the first element name - if it has a subscript, add the appropriate prefix for that number - never use the prefix "mono-" on the first element! · write the second element name - change its ending to "-ide", just like an ionic compound - if it has a subscript, add the appropriate prefix for that number That's it! No criss-cross, no parentheses, no roman numerals. Molecular Compounds Prefixes 6 hexa- 7 hepta- 8 octa- 4 tetra- 9 nona- 5 penta- 10 deca- 1 mono2 3 ditri- Some weird rules to remember: · never use "mono-" on the first element · drop the "a-" when used with elements that start with a vowel - i.e., tetroxide, pentiodide, etc. http://www.kentschools.net/ccarman/ October 12, 2011 CP Chemistry: Naming molecular compounds October 12, 2011 Molecular Compounds Examples SF6 Cl2O7 nitrogen monoxide diiodine pentoxide http://www.kentschools.net/ccarman/