9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Naming Binary Molecular
Compounds
Carbon and oxygen combine to form carbon
monoxide (CO) and carbon dioxide (CO2), but
these two invisible gases are very different.
Slide
1 of 15
© Copyright Pearson Prentice Hall
End Show
9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Naming Binary Molecular
Compounds
Sitting in a room with small amounts of CO2 in
the air would not present any problems. If the
same amount of CO were in the room, you could
die of asphyxiation. A naming system that
distinguishes between these two compounds is
needed.
Slide
2 of 15
© Copyright Pearson Prentice Hall
End Show
9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Naming Binary Molecular
Compounds
A prefix in the name of a binary molecular
compound tells how many atoms of an element
are present in each molecule of the compound.
You MUST know these Prefixes
Slide
3 of 15
© Copyright Pearson Prentice Hall
End Show
9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Naming Binary Molecular
Compounds
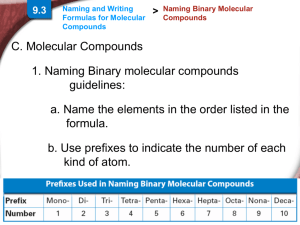
Some guidelines for naming binary molecular
compounds:
• Name the elements in the order listed in the
formula.
• Use prefixes to indicate the number of each
kind of atom.
• Omit the prefix mono- when the formula
contains only one atom of the first element in
the name.
• The suffix of the name of the second element
Slide
4 of 15
is -ide.
© Copyright Pearson Prentice Hall
End Show
9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Writing Formulas for Binary
Molecular Compounds
Writing Formulas for Binary Molecular
Compounds
How do you write the formula for a binary
molecular compound?
Use the prefixes in the name to tell you
the subscript of each element in the
formula. Then write the correct symbols
for the two elements with the appropriate
subscripts.
Slide
5 of 15
© Copyright Pearson Prentice Hall
End Show
9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Writing Formulas for Binary
Molecular Compounds
Silicon carbide is a hard material like diamond.
The name silicon carbide has no prefixes, so the
subscripts of silicon and carbon must be one.
Thus, the formula for silicon carbide is SiC.
Slide
6 of 15
© Copyright Pearson Prentice Hall
End Show
9.3 Section Quiz.
Assess students’ understanding of the
concepts in Section 9.3.
Continue to:
-or-
Launch:
Section Quiz
Slide
7 of 15
© Copyright Pearson Prentice Hall
End Show
9.3 Section Quiz.
1. Which of the following compounds is named
INCORRECTLY?
a. CS2, carbon disulfide
b. BCl3, boron trichloride
c. IF7, iodine heptafluoride
d. PCl5, phosphorus hexachloride
Slide
8 of 15
© Copyright Pearson Prentice Hall
End Show
9.3 Section Quiz.
2. Which of the following molecular compounds
is named INCORRECTLY?
a. SbCl3, antimony trichloride
b. C2O5, dicarbon pentoxide
c. CF4, carbon tetrafluoride
d. H3As, hydrogen arsenide
Slide
9 of 15
© Copyright Pearson Prentice Hall
End Show
9.3 Section Quiz.
3. The correct formula for tetraphosphorus
trisulfide is
a. P3S4
b. S3P4
c. P4S3
d. S4P3
Slide
10 of 15
© Copyright Pearson Prentice Hall
End Show
Practicing Skills: Naming Chemical Compounds
Practicing Skills: Naming Chemical Compounds
How do you use a flowchart to write
the name of a chemical compound?
Follow the arrows and answer the
questions on the flowchart to write
the correct name for a compound.
Slide
11 of 15
© Copyright Pearson Prentice Hall
End Show
Practicing Skills: Naming Chemical Compounds
For names of acids
see regents ref
tables
Slide
12 of 15
© Copyright Pearson Prentice Hall
End Show
9.5
a. CuSO4 is an example from the flowchart.
The compound will end in -ite or -ate. Cu is
not part of Group A, so you must name the
ions and use a Roman numeral to identify
the charge of the transition metal. The
name is copper(II) sulfate.
Slide
13 of 15
© Copyright Pearson Prentice Hall
End Show
9.4
Practicing Skills: Writing Chemical Formulas
What four guidelines should you
follow to write the formula of a
chemical compound?
Slide
14 of 15
© Copyright Pearson Prentice Hall
End Show
9.5
Practicing Skills: Naming ChemicalCompounds
a. In writing a chemical formula from a
chemical name, it is helpful to remember
the following guidelines.
An -ide ending generally indicates a
binary compound.
An -ite or -ate ending means a
polyatomic ion that includes
oxygen is in the formula.
Slide
15 of 15
© Copyright Pearson Prentice Hall
End Show
Practicing Skills: Naming Chemical Compounds
9.5
Prefixes in a name generally indicate
that the compound is molecular.
A Roman numeral after the name of a
cation shows the ionic charge of
the cation.
Slide
16 of 15
© Copyright Pearson Prentice Hall
End Show
Section Quiz 9.5.
Assess students’ understanding of the
concepts in Section 9.5.
Slide
17 of 15
© Copyright Pearson Prentice Hall
End Show
9.5 Practicing Skills: Naming Chemical Compounds
Use periodic
table for
charges
Use ref tables
for polyatomic
ions
Slide
18 of 15
© Copyright Pearson Prentice Hall
End Show
Section Quiz 9.5.
2. You want to write the chemical formula for
iron(II) chloride. Based on Figure 9.22, after
identifying symbols, what is the correct next
step in the flowchart?
a. Group A elements
b. Roman numerals
c. Balance charges
d. Polyatomic ions
Slide
19 of 15
© Copyright Pearson Prentice Hall
End Show
Section Quiz 9.5.
3. Using the flowchart in Figure 9.20, if you
determine that the name of an ion ends in -ite
or -ate, the ion is a
a. polyatomic cation.
b. polyatomic anion.
c. transition metal cation.
d. group A anion.
Slide
20 of 15
© Copyright Pearson Prentice Hall
End Show