Chapter 2 - CCRI Faculty Web
advertisement

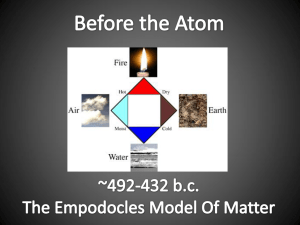
Chapter 2 Ancient Greek and Roman concepts of Nature 1. Continuists – Aristotle 2. Atomists – Leucippus, Democritus & Lucretious Dark & Middle Ages – Little change Late Middle Ages – Reformation & Renaissance • Beginnings of Modern Science – Experimentation and questioning. • Modern concept of elements – Robert Boyle -1661 Two new LAWS: 1. Law of Conservation of Mass ––The total mass of substances during a chemical or physical change does not change. Discovered by Antoine Lavoisier in late 18th Century 2. Law of Definite Proportions – Discovered by Joseph Proust in 1799 – All samples of a compound always have the same ratio of elements no matter where it is obtained from. Explained by English School Teacher, John Dalton Modern Atomic Theory: John Dalton –1803 1. All matter made up from tiny, indestructible, indivisible spheres called atoms. 2. Atoms of an element are all identical. Atoms of different elements are different. 3. Compounds are formed when atoms combine together in small, whole number ratios to form molecules. This ratio is always the same for any compound. 4. A chemical reaction involves a rearrangement of atoms. No atoms are created, destroyed or broken apart in a chemical reaction By now the modern concept of elements was well accepted and many attempts had been made to arrange the known elements in some kind of coherent manner. All attempts had fatal flaws until a Russian chemist, Dmitri Mendeleev, in 1869 devised an approach which is still basically the same that is used today. His Periodic Table was based on 2 ideas: 1. Arrange elements in order of increasing atomic mass 2. Have groups or families of elements Predicted properties of missing elements – for example, germanium: Property Predicted Observed Atomic mass 72 72.6 Density (g/cm3) 5.5 5.47 Color Grayish white Dirty gray