Historical Development of the Atom
advertisement

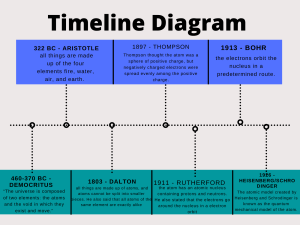
Historical Development of the Atom Explain the contributions made by each of the following in the development of our understanding of the atom. 1. Democritus: first one to state that atoms existed. 2. John Dalton: developed the atomic theory; had a solid sphere as his model—like a billiard ball. 3. J. J. Thomson: discovered the electron; had the plum pudding model—but What does that mean-*think chocolate chip ice cream 4. Ernest Rutherford: performed the gold foil experiment; determined atoms are mostly empty space, have a nucleus which is positively charged and massive 5. Robert Millikan: determined the charge and mass of an electron 6. Neils Bohr: electrons move in orbits around the nucleus—called the Planetary model; stated electrons in atoms have fixed amounts of energy 7. Dmitri Mendeleev: first one to arrange periodic table—done in columns by increasing atomic mass 8. Henry Moseley: arranged periodic table by increasing atomic number 9 Max Planck energy atoms can only change in small fixed amounts known as quanta 10. Werner Heisenberg: developed uncertainty principle- you can know where an electron is or its speed but not both. 11. Louis de Broglie: developed the Quantum mechanical model.