Ch 2 Homework questions
advertisement

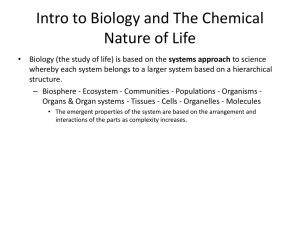
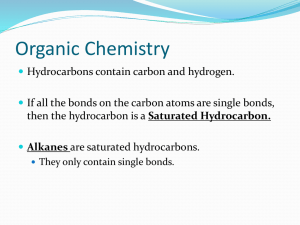
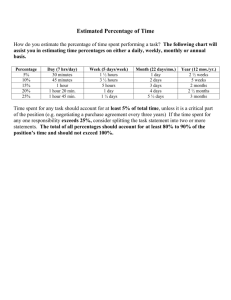
Name ___________________________________ Hour ________ Date _______ Homework – Chapter 2 – Chemistry of Life 1. Atoms can have various numbers associated with them: a) Define the following and show where each of them is placed relative to the symbol of an element such as C: 1. atomic number 2. mass number 3. atomic mass b) Define what is meant by valence number c) Which of these four numbers (structure) is most related to the chemical behavior (function) of an atom? Explain. 2. Various elements are used by living things: a) Why are some elements called macro-elements and others microelements? b) Speculate on whether the survival of an organism would be different if it was deficient in a macro-element versus deficient in a microelement. Explain. 3. What are isotopes and what are their uses in scientific research? 4. Explain what is meant by saying that the sharing of electrons between atoms falls on a continuum from nonpolar covalent bonds to ionic bonds. 5. What is the difference between a molecule and a compound? 6. The percentages of naturally occurring elements making up the human body are similar to the percentages of these elements found in other organisms. How could you account for this similarity among organisms? 7. While waiting at an airport, the senior author of your textbook, Dr. Neil Campbell, once overhead this claim, “It’s paranoid and ignorant to worry about industry or agriculture contaminating the environment with their chemical wastes. After all, this stuff is just made of the same atoms that were already present in our environment”. How would you counter this argument?