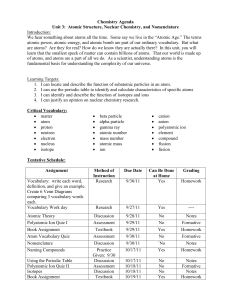
Chapter 3 Study Guide
advertisement
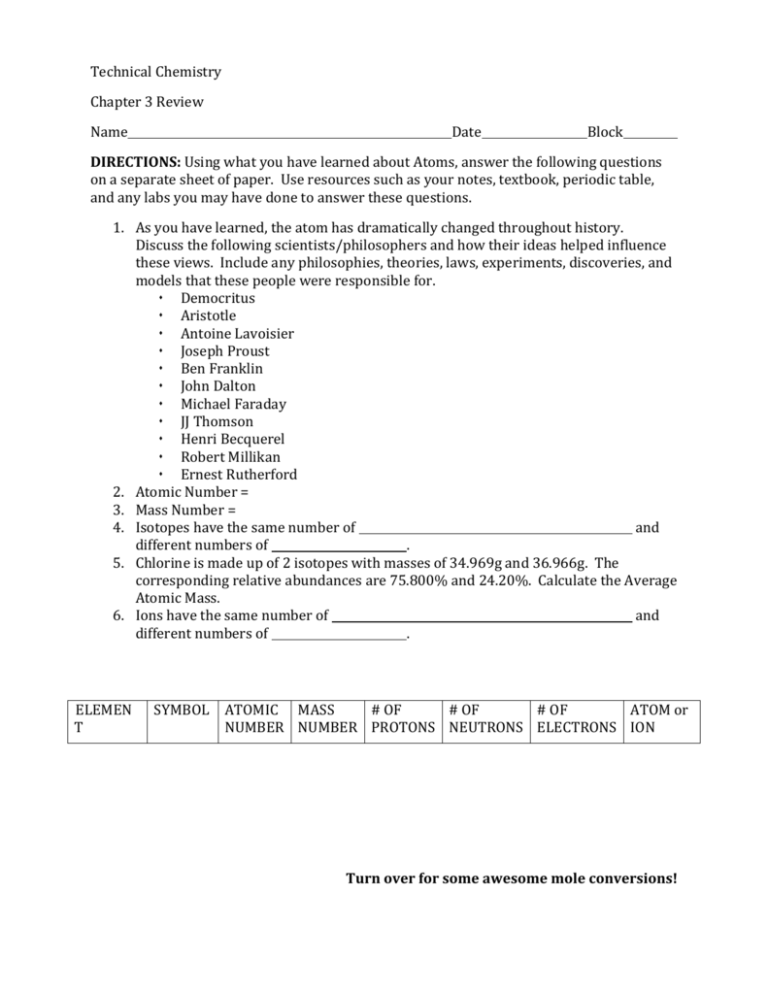
Technical Chemistry Chapter 3 Review Name Date Block DIRECTIONS: Using what you have learned about Atoms, answer the following questions on a separate sheet of paper. Use resources such as your notes, textbook, periodic table, and any labs you may have done to answer these questions. 1. As you have learned, the atom has dramatically changed throughout history. Discuss the following scientists/philosophers and how their ideas helped influence these views. Include any philosophies, theories, laws, experiments, discoveries, and models that these people were responsible for. Democritus Aristotle Antoine Lavoisier Joseph Proust Ben Franklin John Dalton Michael Faraday JJ Thomson Henri Becquerel Robert Millikan Ernest Rutherford 2. Atomic Number = 3. Mass Number = 4. Isotopes have the same number of and different numbers of . 5. Chlorine is made up of 2 isotopes with masses of 34.969g and 36.966g. The corresponding relative abundances are 75.800% and 24.20%. Calculate the Average Atomic Mass. 6. Ions have the same number of and different numbers of . ELEMEN T SYMBOL ATOMIC MASS # OF # OF # OF ATOM or NUMBER NUMBER PROTONS NEUTRONS ELECTRONS ION Turn over for some awesome mole conversions! Bromine 45 26 56 37 +3 ion 79 7. How many grams are in 4.67 moles of Calcium? 8. How many atoms are in 4.67 moles of Calcium? 9. How many grams are in 2.99 x 1025 atoms of Boron? 10. How many atoms are in 33.6g of Magnesium? 197 atom