5.10 Molecular Compounds What are they? How are they formed?
advertisement

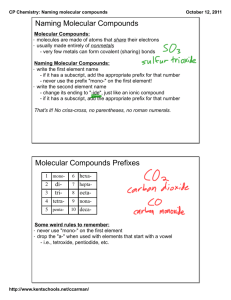
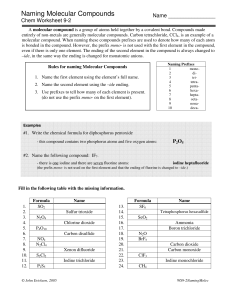
5.10 Molecular Compounds What are they? How are they formed? Molecules and Covalent Bonding • A molecular compound is a pure substance formed from non-metals. • Involves the sharing of electrons between the non-metals in order to obtain full electron orbits. • Examples include: H2O; CO2; H2 • Covalent Bond A shared pair of electrons between two non-metals that hold the atoms together Type of Compounds Diatomic Molecules • Molecules made up of two identical atoms • Some atoms naturally form diatomic molecules • Examples: H2; O2; Br2; N2; F2; Cl2; I2 • These are all gases – hydrogen gas (H2); nitrogen gas (N2); oxygen gas (O2) • YOU MUST MEMORIZE THESE!! Naming Molecular Compounds • When naming molecular compounds, scientists use prefixes to specify the number of each type of element in a molecule. • The prefix mono is used only for the second element in the compound. Prefix Number of atoms mon(o) - 1 di - tri - 2 tetra - 3 4 penta - 5 hexa - 6 hepta - 7 octa - 8 nona - 9 deca - 10 Naming Molecular Compounds 1. Write the name of the first elements and change the ending of the second element to “ide”. 2. Add the prefixes to each element. (NOTE: The prefix mono- is not necessary on the first element.) Example: P2O5 is ________________. Practice 1. N2O5 __________________ 2. SO3 __________________ 3. CS2 __________________ 4. CO __________________ Chemical Formulas for Molecular Compounds 1. Write the symbols for each element in the compound. 2. Use the prefixes for each element to determine how many of each element is found in the molecule and add them to the symbols using subscripts. (NOTE: There will be no prefix in the first element if there is only one.) Practice 1. phosphorus pentabromide _________ 2. carbon tetrachloride _________ 3. diphosphorus trioxide _________ 4. dinitrogen monoxide _________ Common Names • Some molecular compounds are referred to by their common names. • Examples: • H2O – water • NH3 – ammonia • H2O2 – hydrogen peroxide • CH4 – methane • O3 – ozone • NO – nitric oxide • YOU MUST MEMORIZE THESE!!