SNC2P Molecular Compounds FIB
advertisement

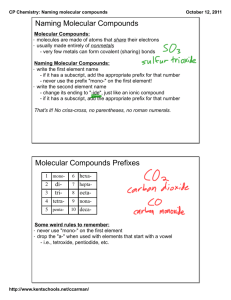
(2.2) MOLECULAR COMPOUNDS (p130-131) Naming and formula writing Molecular Compounds Molecular compounds are formed by combining ________________________________. They do so by ____________________ electrons. The bond formed by sharing electrons is called a _________________________bond. Properties of Molecular Compounds Often soft, _____________________________________________________. If they dissolve in water, they form solutions that ______________________ conduct electricity. Tend to have relatively low ________________________________________. Naming Molecular Compounds Naming molecular compounds is different than naming ionic compounds because ______________________ can ________________________ in many different ways. e.g. carbon and oxygen can combine 2 different ways!!! carbon monoxide CO carbon dioxide CO2 To name a molecular compound are used to show the _________________________ of each type of atom. Prefix system Prefix Number mono 1 di 2 tri 3 tetra 4 penta 5 hexa 6 hepta 7 octa 8 nona 9 deca 10 Examples name formula sulfur trioxide dihydrogen monosulfide SiO2 CF4 N2O5 disulfur dinitride NOTE: never have 2 vowels in a row between the prefix and element ex: 2 “o’s” in monooxide (use monoxide instead) Naming Molecular Compounds A molecular compound has a __________________________ name: first name second name Common Molecular Compounds You must know these common names …. H2O ______________________ H2O2 ___________________________________ CH4 ______________________