alkenes and alkynes - Chemistry at Loyola
advertisement

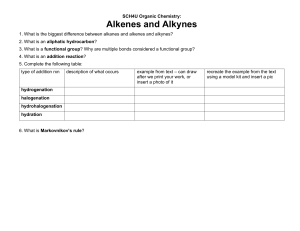
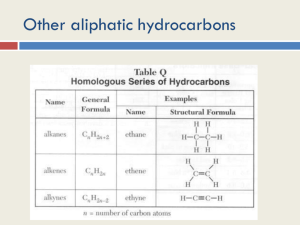
Learning Check … Did I complete all my homework from last class? 0 Are there any homework questions I need help with? 1 2 3 4 5 1.2 Alkenes and Alkynes Alkenes contain one or more double bonds (General formula: CnH2n) Alkynes contain one or more triple bonds (General formula: CnH2n-2) Since alkenes and alkynes are NOT bonded to the maximum possible number of atoms, these compounds are often referred to as unsaturated hydrocarbons Boiling points and melting points: Reactivity: alkynes < alkenes < alkanes alkynes > alkenes > alkanes When naming these molecules, there are a few extra rules to follow: • The main chain must include double or triple bonds at lowest position number (indicate position #) • alkenes end with –ene, alkynes end with -yne • if more than one double bond exists use prefixes (diene, triene, etc) • when numbering the main chain, double and triple bonds have priority over alkyl groups Name the following: (a) C H 3 C H H C HC HC HC 3 3 (b) C H H H H C H 3 C 3 2 C 2 C 2 C C H C H 3 (c) (d) CH3C(CH3)2CH(CH2CH3)CCH (e) Draw the following: (a) 3-methylpent-2-ene (b) 2,5,7-trimethyl-5-propyloct-3-yne Name the following Cyclic Alkene: Draw the following: 3,3-diethyl-2-methylcyclopentene Stereoisomers: Cis and Trans same number atoms, with the double bond in the same position, but with a different 3D geometry around that double bond Cis means that the two groups are on the same side of the double bond Trans indicates that they are on ‘opposite’ sides ex) cis-but-2-ene trans-but-2-ene Self Check How prepared am I to start my homework? Can I … … name and draw alkanes and alkenes … name and draw stereoisomers for an alkene 0 0 1 1 2 2 3 3