2 - Solon City Schools
advertisement

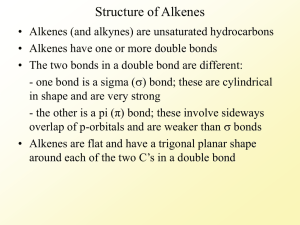
Organic Chemistry Nomenclature: Alkanes Alkenes Alkynes Alkanes – Page 7 Hydrocarbons – made of carbon and hydrogen ◦ Saturated – all single bonds Formula = CnH2n+2 Prefixes for # of Carbons – Page 5 1 Meth 6 Hex 2 Eth 7 Hept 3 Prop 8 Oct 4 But 9 Non 5 Pent 10 Dec Name Methane Ethane Propane Butane 2-Methylpropane Molecular Formula CnH2n+2 Structural Formula Model Summary: IUPAC Rules for Alkane Nomenclature 1. Find and name the longest continuous carbon chain. This is called the parent chain. (Examples: methane, propane, etc.) 2. Number the chain consecutively, starting at the end nearest an attached group (substituent). 3. Identify and name groups attached to this chain. (Examples: methyl-, bromo-, etc.) 4. Designate the location of each substituent group with the number of the carbon parent chain on which the group is attached. Place a dash between numbers and letters. (Example: 3-chloropentane) 5. Assemble the name, listing groups in alphabetical order. The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing. Place a comma between multiple numbers. (Example: 2,3-dichloropropane) Step 1. Find the parent chain. Where is the longest continuous chain of carbons? Step 2. Number the parent chain. Number the parent chain so that the attached groups are on the lowest numbers Methyl is on carbon #2 of the parent chain Methyl is on carbon #4 of the parent chain 1 5 1 8 2 4 3 3 4 2 3 6 7 2 8 1 4 5 5 4 6 3 5 1 GREEN is the right way for this one! 27 1 7 2 6 3 5 Groups on 2 and 5 Groups on 4, 6, and 7 Groups on 2, 3, and 5 4 4 5 3 6 72 1 Groups on 3 and 6 Step 3. Name the attached groups. Carbon (alkyl) groups ◦ Methyl CH3 ◦ Ethyl CH3CH2◦ Propyl CH3CH2CH2 – Step 4. Designate where the group is attached to the parent chain. Use the numbers of the parent chain from step 2 to designate the location of the attached groups to the parent chain. Step 5. Alphabetize the groups, combine like groups, and assemble. The prefixes di, tri, tetra etc., used to designate several groups of the same kind Prefixes are not considered when alphabetizing (Example: dimethyl = m for alphabetizing) Parent chain goes LAST 1,1,1-trichloro-1fluoromethane 1,1-dichloro-1,1difluoromethane Final Names Review Notes – Page 6 (Blank) 1. Name the Parent Chain ◦ With –ane ending 2. Name the Side Chains ◦ With –yl ending 3. Number the Parent Chain ◦ Groups have lowest numbers possible 4. Put It All Together Example 1: 3-ethylheptane Example 2: 2,7-dimethylnonane Example 3: 4-ethyl-2,4,5-trimethyloctane Example 4: 3,3,4,4-tetraethyl-2,2,5,5-tetramethylhexane Name Alkanes – Page 9 Draw Some Simple Alkanes – Page 9 2-methylpentane 2,3-dimethylbutane Homework Review – Pages 17&18 Name 1. dodecane 2. 2-methylheptane 3. 2,3,4-trimethylhexane Structural Formula Cycloalkanes Are ring structures Formula = CnH2n Isomers – Page 19 Draw butane and 2-methylpropane. Isomers – Page 19 Draw pentane, 2-methylbutane, and 2,2dimethylpropane. Alkenes – Page 10 Naming Alkenes – Page 10 Drawing Alkenes – Page 11 1-hexene 2-methyl-4-ethyl-1-nonene Alkynes – Page 12 Naming Alkynes – Page 12 Drawing Alkenes – Page 12 2-hexyne 4-ethyl-1-nonyne Naming Alkenes and Alkynes IUPAC nomenclature rules for alkenes and alkynes are similar to alkanes. Step 1. Name the parent compound. Find the longest chain containing the double or triple bond, and name the parent compound by adding the suffix – ene or –yne to the name of the main chain. Step 2: Number the carbon atoms in the parent chain, beginning at the end nearest to the double or triple bond. If the multiple bond is an equal distance from both ends, begin numbering at the end nearer the first branch point. The number indicates which carbon the multiple bond is AFTER. (i.e. between 2 and 3 is 2-) Step 3: Assign numbers and names to the branching substituents, and list the substituents alphabetically. Use commas to separate numbers, and hyphens to separate words from numbers. Step 4. Indicate the position of the multiple-bond carbon. If more than one multiple bond is present, identify the position of each multiple bond and use the appropriate ending diene, triene, tetraene, and so forth. Step 5. Put it all together. Naming Alkenes and Alkynes When the carbon chain has 4 or more C atoms, number the chain to give the lowest number to the double or triple bond. CH2=CHCH2CH3 CH3CH=CHCH3 CH3C CCH3 Aromatic Compounds – Page 13 In the early days the word aromatics was used to described many fragrant molecules isolated from natural sources. Today the term aromatic is used to describe benzene-like molecules. Benzene is a flat, symmetrical molecule with the molecular formula C6H6. It has alternating three carbon-carbon double and three single bonds. Simple aromatic compounds like benzene are non-polar, insoluble in water, volatile, and flammable. Unlike alkenes, several aromatic hydrocarbons are toxic. Benzene itself is implicated as a cancer causing chemical. Aromatics – Page 13 Benzene’s is C6H6. There are two possible structures with alternating double and single bonds. Naming Aromatics Benzene = Parent Chain Number branches Alkyl Halides – Page 27 Halocarbons - class of organic compounds containing covalently bonded fluorine, chlorine, bromine, or iodine ◦ General formula: R-X (X = halogen) Fluorine Chlorine Iodine Bromine Naming Alkyl Halides