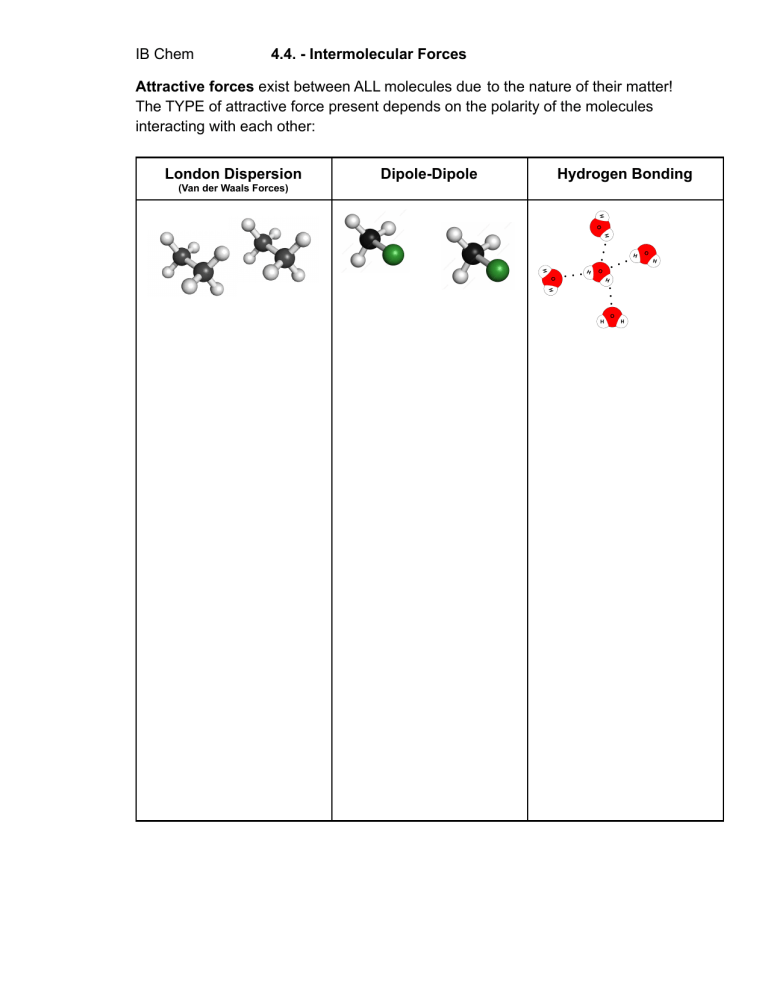
IB Chem 4.4. - Intermolecular Forces Attractive forces exist between ALL molecules due to the nature of their matter! The TYPE of attractive force present depends on the polarity of the molecules interacting with each other: London Dispersion (Van der Waals Forces) Dipole-Dipole Hydrogen Bonding Dipole-induced Dipole Gaseous, non-polar molecules like N2, O2 and CO2 will dissolve slightly in water Let’s talk about melting: Ion-Induced Dipole Allotropes of Carbon Silicon Dioxide