Intermolecular Forces of Attraction
advertisement

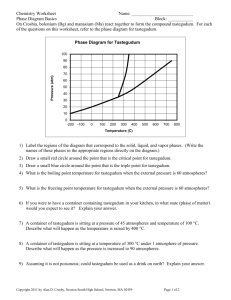
Intermolecular Forces of Attraction 1. Compare using the KMT solids, liquids and gases on the molecular level. 2.The physical properties of a substance depend upon a given substance’s intermolecular forces. A.Vapor Pressure of a substance that exists as a vapor at that temperature. i. the amount i. Inverse relationship Vapor Pressure IMF B. Boiling Point VOLATILE-easy to boil or evaporate i. vapor pressure of a substance equals atmospheric pressure ii. the normal boiling point is when the vapor pressure equals 760 mm Hg or standard atmospheric pressure pentane <butanal <butanol iii. direct relationship boiling point IMF 36°C < 76°C < 118°C C. Heat of Vaporization MM 72, 72 74 g i. the amount of energy needed to turn a specific quantity of liquid to gas. II. direct relationship H vap IMF D. Surface Tension i. water and Hg have strong intermolecular forces that pull the molecules together. E.Viscosity i. resistance to flow ii. Honey, molasses, ketchup 3. Types of intermolecular forces How it Works Factors to Type of molecules Example Type of Force consider Attraction as a result of Polarizability nonpolar Ar, F2 Dispersion Forces Temporary or instantaneous (London Forces) dipoles dipole moment Polar molecules SO2 Dipole-dipole forces Attraction of permanent partial charges the + and – ends of two polar molecules Attractions between a Polar molecules with H2O, HF Hydrogen Bonding hydrogen that is attached to a hydrogen attached to CH3OH –N, -O or –F to another –N, -N, -O, or -F O or -F A. The polarizability of an atom or molecule is influenced by its size and molar mass. i.Polarizability-the ability of an electron cloud to shift or distort position around the nucleus. ii. the larger the surface area, the larger the attractive forces between two molecules and the stronger the intermolecular forces. B. The general trend for relative strengths of intermolecular forces is Hydrogen bonding > dipole-dipole forces > dispersion forces i. remember that this is just a trend so exceptions can occur. ii. all molecules have dispersion forces, but they are only important when there are no other IMF possible. 3. X-ray Diffraction by Crystals A. Bragg Equation n = 2 d sin O i. d = distance between layers or points in the crystal 4. Forces in Solids Type of Solid Example Exist as Type of Attraction NaCl CaO crystal Attraction between ions solidification condensation Properties Strength of Attractive Forces kJ/mole 400 to 4000 Hard, high mp Solid does not conduct Diamond Network of 3-D network of high mp poor 150 to 500 Network atoms covalent bonds conductor SiO2 (Covalent) Individual Attraction Low mp < 100°C 0.05 to 40 H2O, Molecular molecules between poor conductor sugar molecules Cu, Fe Atoms Delocalized Low to high mp 75 to 1000 Metallic held metallic solid good together by bonding conductor of heat or a “sea” electricity of valence electrons A. amorphous solids-without shape or form, solids that lack a three dimensional arrangement of atoms. No sharp melting point or boiling point. i.Examples: glass, rubber, gum, play dough 5. Phase Changes melting vaporization solid liquid gas Ionic 6. To be able to draw and interpret Phase Diagrams A. -summarizes the conditions at which a pure substance exists as a solid, liquid or gas. B. Critical Point i. Critical Temperature, Tc -a temperature above which the gas phase cannot be made into a liquid no matter how large the pressure applied. ii. Critical Pressure, Pc -is the maximum pressure that must be applied to turn a substance into a liquid at the critical temperature. C. Triple Point-all three phases are in equilibrium with each other. D. Water E. Carbon Dioxide Sublimation occurs at normal pressures P R E S S U R E critical point (at constant pressure, follow this type of line) Liquid Solid triple point Temperature Gas (at constant temperature)