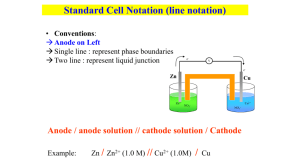
10.1 Galvanic Cells Zn(s) SRA Cu2+(aq) SOA SO42-(aq) H2O(l) Zn(s) SRA Cu2+(aq) SOA SO42-(aq) H2O(l) ▪ When Zn and Cu2+ are in direct contact, we cannot harness the electrical energy of the transferring electrons (it instead gets lost as heat) ▪ In order to harness the electrical energy, the oxidizing agent and the reducing agent have to be separated in order to force the electron transfer through a wire ▪ This electron flow/current can then be used to power electrical devices! ▪ Electric cells adapted for scientific study are often called galvanic cells or voltaic cells ▪ Electric cells adapted for scientific study are often called galvanic cells or voltaic cells ▪ Two electrodes (a solid electrical conductor) are in contact with electrolytes (ions in solution) ▪ The electrolytes surrounding each electrode are separated by a porous boundary that still permits ions to move through tiny openings between the two solutions electrode a solid electrical conductor half-cell an electrode and an electrolyte that form half of a complete cell Zn(s) charge into Zn2+ Cu2+ charge into Cu(s) - e- flow from Zn(s) to Cu(s) creating a current - This stops when there is a buildup of charges in each half cell - Oxidation of Zn (into Zn2+) results in buildup of Zn2+ (+) - Reduction of Cu2+ will decrease Cu2+ ions and only SO42- will be left (-) - The buildup prevents further etransfer → redox reaction stops We can fix this by adding a salt bridge into the system Salt bridge: a u-shaped tube, whose ends are plugged with cotton balls, that is filled with a non-reactive electrolyte. The cotton balls prevent the solution from pouring out, but they are porous to allow ion movement. The flow of ions through the sodium sulfate salt bridge keeps the solution in each half-cell electrically neutral. Na2SO4 Na2SO4 is ideal since it’s soluble and ions do not react with the electrolytes or electrodes SRA (Strong reducing agent) SRA (Strong reducing agent) Na+ migrates to the cathode half cell to offset the loss of Cu2+ ions in order to balance the buildup of Zn2+ Because of the salt bridge, the solutions in each half cell remain electrically neutral ▪ A galvanic cell consists of two half-cells separated by a porous boundary with solid electrodes connected by an external circuit to produce an electrical current. ▪ The cathode is the positive electrode. Reduction of the strongest oxidizing agent present in the cell occurs at the cathode ▪ The anode is the negative electrode. Oxidation of the strongest reducing agent present in the cell occurs at the anode ▪ Electrons travel in the external circuit from the anode to the cathode ▪ Internally, anions in the salt bridge move toward the anode and cations move toward the cathode as the cell operates. The solution remains electrically neutral. Cell Notation ▪ A cell can be represented with a shorthand called line notation: ▪ The single line (|) indicates a phase boundary such as the interface of an electrode and an electrolyte in a half-cell ▪ The double line (||) to indicate a salt bridge and represents a physical boundary such as a porous boundary between half-cells Zn(s) Zn2+(aq) Cu2+(aq) SO42-(aq) Na+(aq) Cu(s) Cell Potential the electric potential difference (voltage) between the two half-cells in a galvanic cell; the SI unit is the volt, and the unit symbol is V (1 V 5 1 J/C) Ultimately, can electricity potentially be produced How do we calculate for it? Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s) ∆E°r (cell) = E°r (cathode) – E°r (anode) standard cell potential (∆E°r (cell) the electric potential difference of a galvanic cell that is operating under standard conditions standard reduction potential (E°r) the ability of a half-cell to attract electrons in a cell that is operating under standard conditions Standard Cells and Standard Cell Potential Because cell potential varies depending on the concentration of the chemicals in a cell. To study cells more easily, chemists have defined standard conditions under which cells operate. A standard cell is a galvanic cell in which all the entities are at SATP, with a concentration of 1.0 mol/L for solutions. Standard Hydrogen Half Cell How was this developed Everything is according to H+ ΔE°r(cell) and Spontaneity Spontaneous Reaction, ΔE°r(cell)> 0 Positive value means that cell reaction occurs spontaneously (on its own) Reaction at Equilibrium, ΔE°r(cell)= 0 Cell has been used up and no more electrons can be transferred. To continue functioning, cell must be recharged. Non-spontaneous reaction, ΔE°r(cell)<0 Negative value means that cell reaction does not occur spontaneously and need an external source to apply energy for cell reaction to occur Example: Determine the standard cell potential and the net ionic equation for a redox reaction that involves silver and zinc half-cells.