Oxidation & Reduction Electrochemistry
advertisement

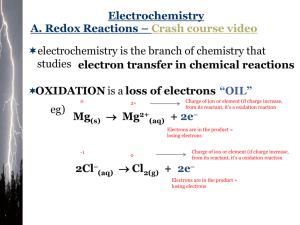
Oxidation & Reduction Electrochemistry BLB 11th Chapters 4, 20 Chapter Summary Oxidation and Reduction (redox) – introduced in chapter 4 Oxidation Numbers Electron-transfer Balancing redox reaction Electrochemical cells Corrosion Electrolysis 20.1, 4.4 Oxidation-Reduction Reactions Oxidation Loss of electrons Increase in oxidation number Gain of oxygen or loss of hydrogen Reduction Gain of electrons Decrease in oxidation number Loss of oxygen or gain of hydrogen Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) Oxidizing agent or oxidant – reactant that contains the element being reduced; is itself reduced Reducing agent or reductant – reactant that contains the element being oxidized; is itself oxidized Oxidation Numbers (p. 132) Assign according to the following order: Atoms zero (since neutral) Ions equal to charge of the ion Nonmetals 1. 2. 3. 4. O H F X −2 +1 (when bonded to other nonmetals) −1 (when bonded to metals) −1 −1 except when combined with oxygen Sum of the oxidation numbers equals zero or the charge of the polyatomic ion. Oxidation numbers practice 1. O2 6. CH2O 2. CH4 7. Cu2+ 3. NO3¯ 8. OCl¯ 4. CH3OH 5. Cr2O72- Redox Reactions Combustion, corrosion, metal production, bleaching, digestion, electrolysis Metal oxidation Activity Series (Table 4.5, p. 136) Some metals are more easily oxidized and form compounds than other metals. Displacement reaction – metal or metal ion is replaced through oxidation A + BX → AX + B 20.2 Balancing Redox Reactions Goal: Balance both the atoms and the electrons Examples: Al(s) + Zn2+(aq) → Al3+(aq) + Zn(s) MnO4¯(aq) + Cl¯(aq) → Mn2+(aq) + Cl2(g) The Rules (p. 830-1) In acidic solution: 1. Divide equation into two half-reactions (ox and red). 2. Balance all elements but H and O. 3. Balance O by adding H2O. 4. Balance H by adding H+. 5. Balance charge by adding electrons (e-). 6. Cancel out electrons by integer multiplication. 7. Add half reactions & cancel out. 8. Check balance of elements and charge. MnO4¯(aq) + Cl¯(aq) → Mn2+(aq) + Cl2(g) CH3OH(aq) + Cr2O72-(aq) → CH2O(aq) + Cr3+(g) The Rules (p. 833) In basic solution: Proceed as for acidic solution through step 7. 8. Add OH¯ to neutralize the H+. (H+ + OH¯ → H2O) 9. Cancel out H2O. 10. Check balance of elements and charge. Cr(s) + CrO4¯(aq) → Cr(OH)3(aq) 20.3 Voltaic Cells A spontaneous redox reaction can perform electrical work. The half-reactions must be placed in separate containers, but connected externally. This creates a potential for electrons to flow. Reactant metal is the most reactive; product metal the least. Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) Line notation: Zn(s)|Zn2+(aq)||Cu2+(aq)|Cu(s) 20.3 Voltaic Cell Net reaction: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) Movement of Electrons e¯ Zn(s) → Zn2+(aq) + 2 e¯ Cu2+(aq) + 2 e¯ → Cu(s) Net reaction: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) 20.4 Cell Potentials Under Standard Conditions EMF – electromotive force – the potential energy difference between the two electrodes of a voltaic cell; Ecell; measured in volts E°cell – standard cell potential (or standard emf) For the Zn/Cu cell, E°cell = 1.10 V electrical work = Coulombs x volts J=CxV J V C Standard Reduction (Half-cell) Potentials E° - potential of each half-cell E°cell = E°cell(cathode) - E°cell(anode) For a product-favored reaction: ΔG° <0 E°cell > 0 Measured against standard hydrogen electrode (SHE); assigned E° = 0 V. App. E, p. 1064 More E° values Mg 2 (aq ) 2e - Mg(s) - 2.37 V Problem Voltaic cell with: Al(s) in Al(NO3)3(aq) on one side and a SHE on the other. Sketch the cell, determine the balance equation, and calculate the cell potential. Problem Voltaic cell with: Pb(s) in Pb(NO3)2(aq) on one side and a Pt(s) electrode in NaCl(aq) with Cl2 bubbled around the electrode on the other. Sketch the cell, determine the balance equation, and calculate the cell potential. 20.5 Free Energy and Redox Reactions n = # moles of e¯ transferred ΔG° = wmax = −nFE° F = 96,485 C/mol (Faraday constant) wmax = max. work ΔG° < 0 E°cell > 0 ΔG° for previous problems 20.6 Cell Potentials Under Nonstandard Conditions Concentrations change as a cell runs. When E = 0, the cell is dead and reaches equilibrium. Nernst equation allows us to calculate E under nonstandard conditions: J R 8 . 3145 RT mol K E E ln Q C F 96,485 mol nF @ 298 K 0.0257 E E ln Q or n 0.0592 E E log Q n Concentration Cells A cell potential can be created by using same half- cell materials, but in different concentrations. Problem 69 Problem 69 Cell EMF and Equilibrium When E = 0, no net change in flow of electrons and cell reaches equilibrium. 0.0257 0.0592 E ln K or E log K n n and nE nE ln K or log K 0.0257 0.0592 K of previous problems 20.7 Batteries and Fuel Cells Batteries self-contained electrochemical power source More cells produce higher potentials Primary – non-rechargeable (anode/cathode) Alkaline: Zn in KOH/MnO2 Secondary – rechargeable (anode/cathode) Lead-acid: Pb/PbO2 in H2SO4 nicad: Cd/[NiO(OH)] NiMH: ZrNi2/[NiO(OH)] Li-ion: C(s,graphite)/LiCoO2 Hydrogen Fuel Cells Convert chemical energy directly into electricity Fuel and oxidant supplied externally continuously Products are only electricity and water cathode: anode: overall: O2(g) + 4 H+(aq) + 4 e¯ → 2 H2O(l) 2 H2(g) → 4 H+(aq) + 4 e¯ 2 H2(g) + O2(g) → 2 H2O(l) PEM Fuel Cell ► 20.8 Corrosion RUST! Anode: M(s) → Mn+(aq) + n e¯ Cathode: O2(g) + 4 H+(aq) + 4 e¯ → 2 H2O(l) or: O2(g) + 2 H2O(l) + 4 e¯ → 4 OH¯ (aq) Preventing Corrosion Anionic inhibition painting oxide formation coating Cathodic inhibition sacrificial anode – attach a metal (like Mg) more easily oxidized galvanizing steel – coating with zinc 20.9 Electrolysis Electrical energy chemical change Hall-Héroult Process for Al Production C(s) + 2 O2-(l) → CO2(g) + 4 e¯ 3 e¯ + Al3+(l) → Al(l)