Oxidation & Reduction Electrochemistry
advertisement

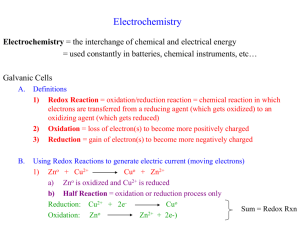
Oxidation & Reduction Electrochemistry BLB 10th Chapters 4, 20 Lab Summary Oxidation and Reduction (redox) Activity series with three metals (Zn, Mg, Cu) Writing equations for reactions Electrochemical cells – standard & non-standard (Assemble for E° > 0.) Writing equations for cells (Reactant metal is the most reactive; product metal the least.) E° calculation (cathode – anode) 21.1, 4.4 Oxidation-Reduction Reactions Oxidation Loss of electrons Increase in oxidation number Gain of oxygen or loss of hydrogen Reduction Gain of electrons Decrease in oxidation number Loss of oxygen or gain of hydrogen Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) Oxidizing agent or oxidant – reactant that contains the element being reduced; is itself reduced Reducing agent or reductant – reactant that contains the element being oxidized; is itself oxidized Redox Reactions Combustion, corrosion, metal production, bleaching, digestion, electrolysis Metal oxidation Activity Series (Table 4.5, p. 143) Some metals are more easily oxidized and form compounds than other metals. Displacement reaction – metal or metal ion is replaced through oxidation A + BX → AX + B 20.3 Voltaic Cells A spontaneous redox reaction can perform electrical work. The half-reactions must be placed in separate containers, but connected externally. This creates a potential for electrons to flow. Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) Line notation: Zn(s)|Zn2+(aq)||Cu2+(aq)|Cu(s) Movement of Electrons e¯ Zn(s) → Zn2+(aq) + 2 e¯ Cu2+(aq) + 2 e¯ → Cu(s) Net reaction: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) 20.4 Cell EMF EMF – electromotive force – the potential energy difference between the two electrodes of a voltaic cell; Ecell; measured in volts E°cell – standard cell potential (or standard emf) For the Zn/Cu cell, E°cell = 1.10 V electrical work = Coulombs x volts J=CxV J V C Standard Reduction (Half-cell) Potentials E° - potential of each half-cell E°cell = E°cell(cathode) - E°cell(anode) For a product-favored reaction: ΔG° <0 E°cell > 0 Measured against standard hydrogen electrode (SHE); assigned E° = 0 V. Problem 28 Sketch and calculate the E°. Voltaic cell with: Al(s) in Al(NO3)3(aq) on one side and a SHE on the other. 20.5 Spontaneity of Redox Reactions n = # moles of e¯ transferred ΔG° = wmax = −nFE° F = 96,485 C/mol (Faraday constant) wmax = max. work ΔG° < 0 E°cell > 0 ΔG° for problem 28 20.6 Effect of Concentration on Cell EMF Concentrations change as a cell runs. When E = 0, the cell is dead and reaches equilibrium. Nernst equation allows us to calculate E under nonstandard conditions: J R 8 . 3145 RT mol K E E ln Q C F 96,485 mol nF @ 298 K 0.0257 E E ln Q or n 0.0592 E E log Q n Cell EMF and Equilibrium When E = 0, no net change in flow of electrons and cell reaches equilibrium. 0.0257 E ln K n and nE ln K 0.0257 K of problem 28 or or 0.0592 E log K n nE log K 0.0592