Objectives: Oxidation
advertisement

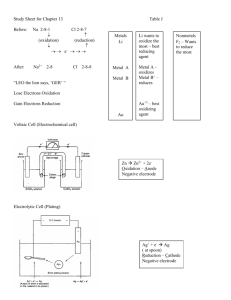
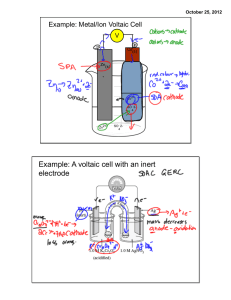
Objectives: Oxidation-Reduction After completing their study of oxidation-reduction, students should be able to: 1. define using their own words, and with examples, the terms: oxidation, reduction, redox, oxidizing agent, reducing agent, oxidized, reduced. 2. compare the quantity of energy released from the most vigorous redox reactions to that released from the most vigorous acid-base reactions. 3. determine the oxidation numbers of atoms in both reactants and products in simple reactions, identify the oxidizing and reducing agents, and identify the substances oxidized and reduced. 4. use the concept of oxidation number together with the concept of a number line to illustrate how oxidation and reduction always occur to the same extent. 5. cite examples in which redox reactions are involved in biochemistry, production of common goods, and environmental changes. 6. balance simple oxidation-reduction equations by at least one method (oxidation number or half-reaction method. Objectives: Electrochemistry Upon completion of their study of electrochemistry, students will be able to: 1. distinguish between anions and cations. 2. define anode and cathode in terms of oxidation and reduction. 3. describe how a voltaic cell produces an internal ionic flow and an external electron flow. 4. write half-cell equations and total equations for voltaic cells. 5. in relation to a voltaic cell, define or explain: anode, cathode, electric potential (volts), salt bridge, internal circuit, external circuit. 6. describe how a battery produces electrical energy. 7. identify the substance being oxidized and the substance being reduced in an electrochemical cell. 8. describe the operation of an electrolytic cell. 9. define cathode, anode, and explain the charge on the cathode and anode in an electrolytic cell. 10. explain similarities and differences between voltaic and electrolytic cells. 11. explain the operation of an apparatus for electroplating with metals. 12. describe the zero-potential hydrogen half-cell. 13. given a table of standard reduction potentials: determine whether a redox reaction will occur; predict the electric potential of a voltaic cell made from two different half-cells; predict the products of an electrolysis reaction. 14. given the reduction potential of one half-cell and the electric potential of a voltaic cell, calculate the reduction potential of the other half-cell.