Chapter 7 figures
advertisement

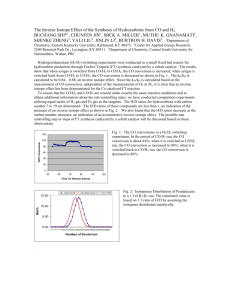
Fig. 7.1. A chemical diagram showing why fractionation occurs when bonds are broken in kinetic reactions. Bonds are often compared to springs, with light isotope bonds depicted as the less massive, easier-to-break springs. Light isotope bonds are slightly wider and have more potential energy than heavy isotope bonds. Adding equal energy to both kinds of bonds results in more rapid bond breaking for the light isotope bonds that need less energy climb out of the energy well as they elongate and break. When bonds are broken, atoms move apart to the right in this diagram, and interatomic distance increases. Bonds are only stable within the energy well. See text for further explanation. Reprinted with permission from Bigeleisen, J. 1965. Chemistry of isotopes. Science 147:463-471. Copyright 1965, AAAS. Fig. 7.2. Carbon isotope compositions of different biochemical fractions of corn (from Fernandez et al. 2003). The percentages of the three fractions lignin, cellulose and the residue add up nicely to 100%, and the isotope mass balance is also very good, better than 0.1o/oo agreement between the total and the summed fractions. Fig. 7.3. Comparison of closed and open systems that are important settings for isotope fractionation. Rxn = reaction. Fig. 7.4. Isotope dynamics in closed systems. Equations are those derived in the next section 7.3 and Technical Supplement 7B on the accompanying CD.. Isotope fractionation is 30o/oo in the illustrated example, and leads to lighter products (lower d) and by difference, heavier substrates (higher d). There are two products, one that accumulates over time (accumulated product) and one that is transient, forming instant-by-instant in time (instantaneous product). Isotopes in the instantaneous product track the substrate isotopes offset by the fractionation factor D, while mass balance imparts a more gradual trend to the isotope values of the accumulated product. See text and Section 7.2 for further details. Fig. 7.5. Isotope dynamics in open, flow-through systems where reactions are split or branched. Isotope fractionation is 30o/oo in the illustrated example, and leads to lighter products (lower d) and by difference, heavier substrates (higher d). See text and Section 7.8 for further details. Fig. 7.6. Comparison of isotope dynamics in closed and open systems. For simplicity, the instantaneous product of the closed system (see Fig. 7.4) has been omitted. Fig. 7.7. Example of an open system where inputs = outputs. Because of this balance, open systems are steady state systems at (or near) equilibrium. Reaction rates are given in arbitrary units of amount reacted/second. Note that the input flow pressurizes output flows, especially so that substrate enters but also exits. The internal reservoir can be small or large. Substrate flowing past a reaction site and exiting is the characteristic feature of open systems. Fig. 7.8. More examples of open vs. closed system dynamics. Top panel: open system dynamics with a one-time use of substrate. Bottom panel: closed system dynamics develop in a plug-flow reactor when there is multiple, successive use of substrate. Fig. 7.9. Both open and closed system dynamics can occur in some instances. In this example for methane diffusing upwards at a landfill site, methanotrophs (methane-consuming bacteria) poised an aerobic-anaerobic interface can utilize methane essentially once in a flow-through, open system (left panel), or sequentially if methane bubbles lingers near bacterial cells and are slowly consumed (right panel). Arrows to the right represent methane consumption by bacteria. Fig. 7.10. d values expected for residual substrate in a closed system, an open system, and in a 50/50 mixed system that combines both closed and open system components. The fractionation factor is assumed to be 20o/oo for substrate consumption in all cases. Figure 7.11. During a reaction, both heavy and light isotope molecules will disappear from the substrate pool to form product. In a “zero order” reaction, the rate of disappearance is linear, a constant amount at each time step. In a “first order” reaction, the rate of disappearance is a constant percentage, leading to an exponential decrease in substrate amounts. Differences between reaction rates of the heavy and light isotope are exaggerated in this drawing so that lines are farther apart than in reality. Figure 7.12. Magnified view of the first order reaction dynamics shows the very slightly faster loss of light isotope than heavy isotope towards the end of the reaction, between time steps 90 and 100. Differences between the heavy and light isotope are not exaggerated in this slide that represents the cumulative fractionation effect over 90-100 time steps, with more heavy isotope material surviving than light isotope material. Figure 7.13. The faster reaction of light than heavy isotopes shown in Figures 7.11 and 7.12 leaves the shrinking substrate pool relatively enriched in heavy isotopes, and higher d values (o/oo) record this enrichment in an easy-tosee fashion. Figure 7.14. The isotope fractionation factor can be extracted from the reaction dynamics, when data is plotted on an (x,y) basis with x = -ln(concentration or amount) and y = the d value of the remaining substrate. The slope of the line gives the fractionation factor in o/oo units, 20o/oo in this case. Figure 7.15. Mass balance accounting helps us follow reactions in closed systems, with mass balance meaning here that the amount of substrate + product always adds to a fixed total. Or, said another way, as substrate is converted to product, nothing is lost and the total of substrate + product always sums to 100%. Figure 7.16. Mass balance accounting also applies when the x-axis of reaction changes from time (previous figure 7.15) to fraction reacted to form product or fractional extent of reaction (this figure). Expressed on this basis, the changes in substrate and product amounts are linear, rather than exponential as in the previous figure. Figure 7.17. Isotope changes also conform to mass balance during reactions, with faster segregation of light isotopes into product balanced by increased heavy isotope content in the residual substrate. The isotope balance of the system remains constant at the input value of 0o/oo, when the mass balance is the mass-weighted average of (amounts x isotopes) or: dINPUT*massINPUT = dSUBSTRATE*massSUBSTRATE + dPRODUCT*massPRODUCT. Fig. 7.18. As previous Figure 7.17, but with a second product, the “instantaneous” product that forms from substrate but is quickly passed to the total, accumulated product. In some reactions, the instantaneous product can be continuously monitored as a gas sparged out of a reaction vessel. In such cases, the system is closed to substrate (no new substrate is added or exported), but still open to product loss. Fig. 7.19. Mass spectrometer measurements of CO2 gas in a helium carrier stream. Top Panel: The mass spectrometer detectors measure the CO2 amounts, here normalized so that 1 = the maximum CO2 signal at mass 44. The chromatogram is from an actual sample analysis with a 3m GC column. The CO2 has been generated in an elemental analyzer and passed over a gas separating column, or gas chromatography (GC) column, to purify it from other combustion gases. Computer programming integrates the total detector response and subtracts a baseline value to quantify the amount of the CO2 in the peak. Integration proceeds for three measured isotopomers of CO2 at masses 44, 45, and 46. Detector responses for only masses 44 (solid line) and 45 (dashed line) are shown here, with no amplification of the small mass 45 signal so that this peak reflects its true natural abundance of about 1.1% vs. the mass 44 signal. The mass 46 signal is smaller still. Bottom Panel: Isotope changes in the CO2 gas as it passes the mass spectrometer detectors, with heavier components (positive d values) arriving first, followed by lighter components (negative d values). Computers integrate the isotope signals for the entire peak using the separate ion beam measurements made at masses 44 and 45, then use the integrated signals to calculate isotope ratios and d values. The integrated value for the peak shown here is d = 0.95o/oo when the baseline value is used as a reference value of 0o/oo. The peak maximum at 53 seconds has an associated instantaneous d value of 5.2o/oo indicated by the arrow. Fig. 7.20. As Fig. 7.19 Top Panel, but the mass 44 and mass 45 signals have been scaled similarly, so that 1 = maximum peak height in each case. Due to isotope fractionation on the upstream GC column, the mass-45 CO2 (dashed line) moves more quickly than mass-44 CO2 (solid line) so that the mass-45 peak leads at the front and back of the mass-44 peak. This isotoperelated difference in the timing of the peaks is very slight, and has been magnified several-fold in this figure. The faster movement of the mass-45 peak causes the swings in the instantaneous d values shown in Fig. 7.19. Fig. 7.21. Nitrogen isotope values of nitrate as a function of nitrate concentration, and two curves fit to the same data. Data is averaged from Fig. 8 in Mariotti et al. 1988. Fig. 7.22. Derivation of the two curves shown in the Fig. 7.21 – data trends are explained equally well by the two opposite approaches, which involve fractionation (left; see also Sections 7.2 for more details on closed system fractionation) or mixing (right; mixing analyzed with the Keeling plot approach of sections 3.5 and 5.7). Fig. 7.23. Diagram of nitrate dynamics near the equator in the Pacific Ocean. Deep water comes to the surface near 2o S (-2o N), then moves northward. This water is rich in nitrate. As water moves north, the nitrate is used up, fueling plankton blooms and eventually a food web that supports tuna production in the region. Fig. 7.24. Particulate organic nitrogen (PON) forms from nitrate is upwelled equatorial waters, and from nitrogen fixation in waters farther to the north. Some PON sinks out and is removed from the system, while some is used in the local food webs. Fig. 7.25. Isotope dynamics expected for N dynamics depicted in Figs. 7.23, 7.24. Key to symbols: closed diamonds = predicted PON, triangles = sedimentary organic nitrogen d15N from tops of cores in the region, diagonal line = predicted PON from nitrate only, horizontal line = predicted PON from gyre with nitrogen fixation. Closed system expectations for upwelled nitrate lead to the prediction of ever-higher d15N values in PON (diagonal line), but this does not occur. Instead, as nitrate levels approach zero, other N sources become important, in this case N from N-fixation and nitrate recycling in mid-ocean gyres located north of the equator. The isotope values in the northern gyres eventually reflect those of the more abundant N-fixation sources. Sediment values are those measured by Francois and Altabet (1994), and are higher than PO 15N values in the upper ocean presumably because of fractionation while organic matter degrades and settles to the bottom (Saino and Hattori 1980, 1987). The 15N dynamics were calculated with an assumption of 5o/oo as the d15N value of nitrate, a fractionation of D = 5o/oo during nitrate use, and a 3o/oo value for the northern ecosystem PON that reflects strong contributions of N fixation (Dore et al. 2002; see also problem 11, Section 7.14 and the answer in I Chi Workbook 7.11 on the accompanying CD). Fig. 7.26. Sulfate concentrations (left) and isotope compositions (right) in a sediment system that is closed to new inputs (squares) or has new inputs via mixing (circles). Concentration gradients are the same both systems, but isotope profiles differ reflecting added sulfate in the mixing system. (The sulfate reduction rate is higher in the mixing system than in the closed system, but higher loss to sulfate reduction is balanced by higher added inputs, with the net result that the sulfate concentration profile for the mixed system is the same as that of the closed system). Fractionation is 25o/oo during sulfate reduction in both systems, and there is an additional 10o/oo diffusive fractionation in the mixing system (see also problem 12, Section 7.14 and the answer in I Chi Workbook 7.12 on the accompanying CD). Fig. 7.27. Two substances A and B have just been brought together and are just beginning an equilibrium exchange reaction. Fig. 7.28. With passage of time, exchange promotes homogenization of isotopes in the two substance of previous Figure 7.27, though there are still slight isotope differences surviving this process, indicated by the milder differences in shadings of the two substances. Fig. 7.29. Equilibrium reactions for the light isotope components ( LA and LB) and heavy isotope components (HA and HB) of the substances A and B involved in an exchange reaction. The rate constants for the reactions are given as kinetic “k” values, with superscripts denoting light (L) and heavy (H) isotope components, and subscripts denoting forwards (F) and reverse (R) reaction directions. Fig. 7.30. Isotope exchange reactions at steady state for two substances A and B, with the reaction dynamics given using the d and D notation. Fig. 7.31. Predicted isotope differences at equilibrium between two types of carbon (C), C in methane and C in CO2, as a function of temperature. Note that isotope differences are largest at colder temperatures. The differences decrease at higher temperature where all C bonds become more fluid and similar. At higher temperatures bonds become more energetic, and small neutron-related isotope differences become less important and less pronounced. From: Urey 1947. Fig. 7.32. Oxygen isotope variations in marine carbonates as a function of temperature. This temperature variation is due to an equilibrium isotope effect between oxygen in water and oxygen in carbonates. Smaller fractionations (indicated by lower d18O values in this case) occur at higher temperatures where bond differences become smaller for the heavy vs. light oxygen isotopes. Key to symbols: Triangles = theoretical calculated values (McCrea 1950), squares = field data. Field data are for marine molluscs (mostly bivalves; Epstein et al. 1953). Paleontologists have used this relationship to estimate temperatures of ancient seas from oxygen isotopes in seashells. Fig. 7.33. Diagram of an open system with only one output. Fig. 7.34. Diagram of an open system with substrate entering a reactor box where product is formed with fractionation and unused substrate exits without further fractionation. See Box 7.5 for details. Fig. 7.35. Diagram of an open system with substrate entering a reactor box where two products are formed with fractionation. See Box 7.5 for details. Fig. 7.36. Graphical results for open system diagrammed in Fig. 7.35, fractionation factor D1>D2. Fig. 7.37. Graphical results for open system diagrammed in Fig. 7.35, but the magnitude of the fractionation factor is reversed from D1>D2 (results shown in Fig. 7.36) to D2>D1 (results shown here). Fig. 7.38. Initial diagram for carbon isotope fractionation during C 3 photosynthesis. CO2 diffuses into the plant stomata and can be fixed by the plant or diffuse back out. See Box 7.6 for details. Fig. 7.39. Carbon isotope fractionation during C3 photosynthesis, continued from Fig. 7.38. Mass balance helps budget the isotope values, starting with fractional accounting. The fraction fixed by the plant is f, and 1-f is the fraction diffusing back out. Fractionations associated with these fluxes are 29o/oo and 4.4o/oo respectively (O’Leary 1988). With these values, mass balance algebra for this open system allows calculation of the -28.4o/oo isotope value of CO2 being fixed. See Box 7.6 for further details Fig. 7.40. Results for the photosynthesis example, d vs. f with f = fraction CO2 reacted = 0.35, a typical value for C3 plants. See Box 7.6 for further details. Fig. 7.41. Generalized open system model for one box that has one entry flux and two exit fluxes (top panel). The starting material lies outside the box and is an assumed reference material with d = 0o/oo that does not change in this reaction. Material fluxes in and out, with a fraction “f” of the influx forming product, and the remaining fraction “1 – f” fluxing out as efflux. Fractionations (D) associated with influx to the box and efflux from the box are assumed to be 0o/oo, but there is a strong fractionation (maximum = 100 units) associated with the consumption step where material is removed to form product via bond formation. In this system, the observed or net isotope fractionation during product formation can approach zero when little material exits the system via diffusion or advection (middle panel), or when supply from the entry flux is much less than demand represented by the flux to form product (bottom panel). Maximal fractionations are expected when most entering material leaves the system unconsumed (middle panel) and when entry supply greatly exceeds consumption demand (bottom panel). Note that the bottom two panels have different x-axes both related to f, the fraction of material that is forming product (top panel). The middle panel shows the net fractionation D values observed in the product as a function of 1 – f, while the bottom panel shows the net fractionation D values as a function of 1/f. The possible range in f is 0 to 1. 7.42. Sulfur isotope variations in sulfides become larger as earth evolves over time, with the geological time line running from right to left in this graph. The increased isotope variations reflect increasing importance of bacterial sulfate reduction from 3.5 billion years ago, and build-up of oxygen in the atmosphere in more recent times. The shaded area between the two top lines represents isotope compositions of sulfate, while the single bottom line represents a hypothetical maximum fractionation for sulfides with isotope values offset 55o/oo lower than sulfate isotope values. (From Canfield 1998; used with the permission of the author and Nature Publishing Group. Copyright 1998). 7.43. Diagram of an aquatic sulfuretum in which microbial partners oxidize and reduce sulfur in a complete cycle. Sulfur oxidizing bacteria (SOBs) oxidize sulfides to sulfate with light or oxygen, while sulfate reducing bacteria (SRBs) reverse the process, reducing sulfate to sulfides. The microbial flora influences the sulfur compositions of sediments, especially adding sulfides via reaction with iron and organic matter. Detrital sulfur plants would be the sole source of sedimentary sulfur if SRBs were not generating sulfides. RIS = reduced inorganic sulfur, CRS = chromium reducible sulfur. 7.44. The process of bacterial sulfate reduction requires both sulfate and an energy source, labile carbon. Sulfate reduction is actually a kind of respiration. In the absence of free oxygen (O 2), bacteria use the 4 oxygens in sulfate instead. A byproduct of this anaerobic respiration is sulfide that can accumulate in sediments. 7.45. A laboratory sulfuretum involving Desulfovibrio vulgaris, a sulfate reducing bacterium, and Chlorobium phaeobacteroides, a photosynthetic bacterium that oxidizes elemental sulfur (S o) and sulfide. So is an important intermediate in sulfur cycling in this sulfuretum, and will accumulate inside the Chlorobium. From Fry et al. 1988. 7.46. Sulfur cycling in a laboratory sulfuretum with both sulfate reducing bacteria and sulfide oxidizing photosynthetic bacteria. Top panel: The dark bar indicates dark conditions where only sulfate reduction was happening, then lights were turned on and photosynthetic bacteria rapidly oxidized sulfide to elemental sulfur (So) and finally to sulfate, completing the S cycle. Thiosulfate (S2032-) appeared transiently as part of the sulfur cycling near the end of the experiment. Middle panel: Accumulation of So inside the photosynthetic bacteria increased the opacity or optical density of the culture after lights were turned on. Bottom panel, large circle: During bacterial reduction of sulfate, isotopes changed in accordance with a normal kinetic isotope effect, with light S (d34S <0o/oo) accumulating in the sulfide pool (SS2-) while heavy S (d34S >0o/oo) accumulated in the residual sulfate (SO42-) pool. Bottom panel, smaller circle: Starting at 44 hours when lights were turned on, sulfide was rapidly oxidized to So, and isotopes changed in an apparently reversed or “inverse” manner, with light S accumulating in the residual sulfide pool and heavy S accumulating in the So product. This apparent inverse isotope effect was actually the result of a fast equilibrium isotope effect between two sulfide pools, with So formed from the heavier of the two sulfide pools. See text for further explanation. From Fry et al. 1988. Used with permission, American Society of Microbiology. Fig. 7.47. Summary steady state model for isotope distributions in a model laboratory sulfuretum (Fry et al. 1988). Laboratory experiments were never at steady state or equilibrium where all reaction rates would be equal and inputs = outputs by mass and by isotopes for each of the three pools. Fractionation factors are e values associated with closed system equations. e values are permil fractionation factors similar to D values, but with opposite sign (in these examples, this approximation holds: e = -D). Used with permission, American Society of Microbiology. Fig. 7.48. Calculating fractionation factors from laboratory culture data. Right panel: Sulfate reducing bacteria consume sulfate and produce sulfides with a normal kinetic isotope effect. Light isotopes react faster and concentrate in the product sulfide, leaving heavy isotopes in the residual sulfate. Right panel: The fractionation factor e = -6.5o/oo (D = 6.5o/oo) is calculated as the slope of the straight line using the closed system equations of Mariotti et al. (1981), where data are (ln(f), d34S) for the residual sulfate and (f*ln(f)/(f-1), d34S) for the product sulfide, with f = fraction of unreacted substrate. From Fry et al. 1988. Used with permission, American Society of Microbiology. Fig. 7.49. A conceptual supply-demand model of sulfur isotope fractionation by bacteria in sediments. Both sulfate and carbon are important controls of bacterial sulfate reduction, with sulfate representing the oxygen supply and carbon representing the oxygen demand. Fractionation during sulfate reduction to form sulfides is maximal when sulfate supplies are high and carbon supplies low. Lakes generally have low concentrations of sulfate vs. marine systems, <100 vs. 28,000 mmol m-3 respectively. The smaller sulfur isotope fractionation in lake vs. marine sediments is likely due to the much lower sulfate levels in lakes, but may be due also in part to higher supplies of easily used labile carbon. Fig. 7.50. Carbon isotope differences between plants and lipids from those plants (from Park and Epstein 1961). Data are consistent with open system fractionation of about 9o/oo during lipid synthesis. Fig. 7.51. Isotope differences for two carbon pools important in the biogeochemical evolution of the earth’s biosphere. Biology and especially plants convert inorganic carbonate carbon (top line) into organic carbon that is preserved in rocks as total organic carbon (TOC, bottom line). The earth’s biosphere behaves as a giant open system through geological time, with volcanoes adding inorganic carbon, and plants and sediments sequestering this carbon. In the far distant past, the biosphere operated at a low value of “f” (the fraction of carbon reacted and stored in the sediments, about 0.12, left vertical line), a less productive time when more carbon remained in the inorganic carbonate pool. More recently, the biosphere but has upshifted to a higher value of “f” (about 0.19, right vertical line), consistent with higher oxygenation of the biosphere. See text for further details. From: Schopf, J. William; Earth’s Earliest Biosphere. Copyright 1983. Princeton University Press. Reprinted by permission of Princeton University Press.