The Inverse Isotope Effect of the Synthesis of Hydrocarbons from CO
advertisement
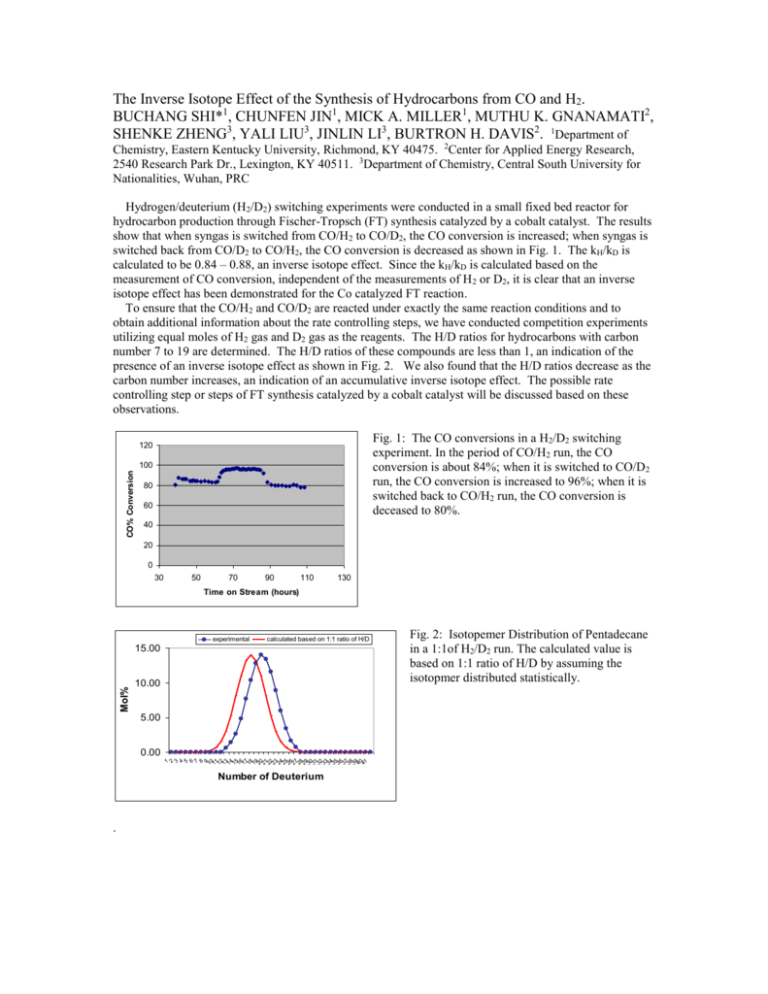
The Inverse Isotope Effect of the Synthesis of Hydrocarbons from CO and H2. BUCHANG SHI*1, CHUNFEN JIN1, MICK A. MILLER1, MUTHU K. GNANAMATI2, SHENKE ZHENG3, YALI LIU3, JINLIN LI3, BURTRON H. DAVIS2. 1Department of Chemistry, Eastern Kentucky University, Richmond, KY 40475. 2Center for Applied Energy Research, 2540 Research Park Dr., Lexington, KY 40511. 3Department of Chemistry, Central South University for Nationalities, Wuhan, PRC Hydrogen/deuterium (H2/D2) switching experiments were conducted in a small fixed bed reactor for hydrocarbon production through Fischer-Tropsch (FT) synthesis catalyzed by a cobalt catalyst. The results show that when syngas is switched from CO/H2 to CO/D2, the CO conversion is increased; when syngas is switched back from CO/D2 to CO/H2, the CO conversion is decreased as shown in Fig. 1. The kH/kD is calculated to be 0.84 – 0.88, an inverse isotope effect. Since the kH/kD is calculated based on the measurement of CO conversion, independent of the measurements of H 2 or D2, it is clear that an inverse isotope effect has been demonstrated for the Co catalyzed FT reaction. To ensure that the CO/H2 and CO/D2 are reacted under exactly the same reaction conditions and to obtain additional information about the rate controlling steps, we have conducted competition experiments utilizing equal moles of H2 gas and D2 gas as the reagents. The H/D ratios for hydrocarbons with carbon number 7 to 19 are determined. The H/D ratios of these compounds are less than 1, an indication of the presence of an inverse isotope effect as shown in Fig. 2. We also found that the H/D ratios decrease as the carbon number increases, an indication of an accumulative inverse isotope effect. The possible rate controlling step or steps of FT synthesis catalyzed by a cobalt catalyst will be discussed based on these observations. Fig. 1: The CO conversions in a H2/D2 switching experiment. In the period of CO/H2 run, the CO conversion is about 84%; when it is switched to CO/D2 run, the CO conversion is increased to 96%; when it is switched back to CO/H2 run, the CO conversion is deceased to 80%. 120 CO% Conversion 100 80 60 40 20 0 30 50 70 90 110 130 Time on Stream (hours) experimental calculated based on 1:1 ratio of H/D Mol% 15.00 10.00 5.00 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 0.00 Number of Deuterium . Fig. 2: Isotopemer Distribution of Pentadecane in a 1:1of H2/D2 run. The calculated value is based on 1:1 ratio of H/D by assuming the isotopmer distributed statistically.