Worksheet: Atoms
advertisement

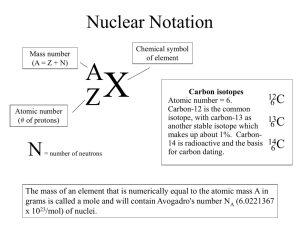
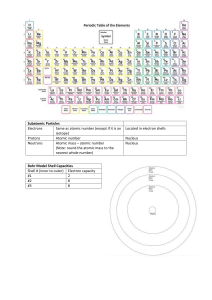
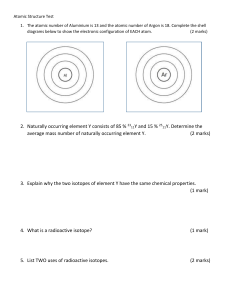
Worksheet: Atoms (25-pts) 1. What are the names and charges of the three subatomic particles? 2. What two parts of an atom make up the nucleus? 3. What is the name of the particle that orbits the nucleus? How big is it? 4. Why are the electrons also called an “electron cloud?” 5. In 1871, what did Ernest Rutherford find out about the atom? 6. What is an element? 7. How many different kinds of elements exist? How many of these are common? 8. How many elements occur naturally in the earth? 9. What is meant by “atomic number” and “mass number?” 10. In the diagram, what do the “20” and “40” represent? 20 Ca 40 11. What is an isotope? 12. What do all isotopes of hydrogen have in common with each other? 13. Now many neutrons are in an isotope of silver with a mass number of 182 and atomic number of 79? 14. Using the periodic table on appendix-24 in your book, list the symbol and atomic number of these elements: Carbon, Sodium, Oxygen, Gold, Uranium