Organic Chemistry - Moorpark College Home Page
advertisement
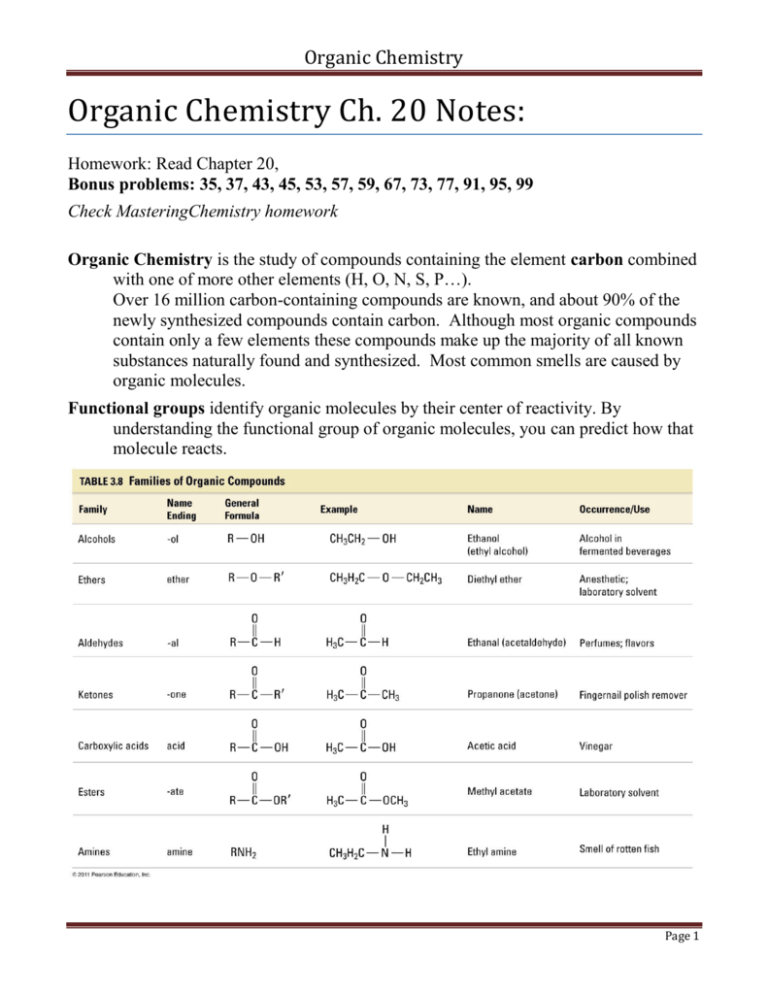
Organic Chemistry Organic Chemistry Ch. 20 Notes: Homework: Read Chapter 20, Bonus problems: 35, 37, 43, 45, 53, 57, 59, 67, 73, 77, 91, 95, 99 Check MasteringChemistry homework Organic Chemistry is the study of compounds containing the element carbon combined with one of more other elements (H, O, N, S, P…). Over 16 million carbon-containing compounds are known, and about 90% of the newly synthesized compounds contain carbon. Although most organic compounds contain only a few elements these compounds make up the majority of all known substances naturally found and synthesized. Most common smells are caused by organic molecules. Functional groups identify organic molecules by their center of reactivity. By understanding the functional group of organic molecules, you can predict how that molecule reacts. Page 1 Organic Chemistry Solubility: Most organic compounds are nonpolar (the C-C and C-H bonds have low electronegativity difference) so these compounds are soluble in nonpolar solvents and not polar water. Some organic compounds have a polar area (C-O or C-N) and if the molecule is small, will be soluble in water. Most organics are neutral, the acidic organic compounds include carboxylic acids and the basic organic compounds include amines. Hydrocarbons are organic compounds containing only C and H and include alkanes, alkenes, alkynes, and aromatics. These compounds are nonpolar. The intermolecular attractions are London Dispersion Forces so the boiling points are relatively low and increase as the molecular weight is increased. All hydrocarbons undergo combustion reactions with oxygen in air. Page 2 Organic Chemistry Compounds: Organic compounds are easily decomposed into simpler substances by heating, but inorganic substances are not. Inorganic compounds historically were readily synthesized in the lab, but synthesis of organic compounds in the lab is harder. Carbon is unique: Carbon can bond to as many as four other atoms. Covalent bonds to carbon are very strong and nonreactive. Carbon atoms can attach together in long chains. Carbon atoms can attach together to form rings. Carbon atoms can form single, double, or triple bonds. Isomers: different molecules with the same molecular formula. Structural isomers attach atoms in a different pattern. Stereoisomers have the same atom attachments, but different spatial orientation; geometric stereoisomers are cis and trans, while optical stereoisomers (also called enantiomers) have a chiral carbonattached to 4 different items and make nonsuperimposeable mirror images. ALKANES: The general formula for an alkane is CnH2n+2 Alkanes contain only single bonds and are saturated hydrocarbons. Methane is the simplest alkane containing only 1 carbon. CH4 has a tetrahedral structure with angles of 109.5°. The term “normal” alkane represents that the carbons are all in a row, the first carbon is attached to the second and so on without branching in various directions. The common reaction with alkanes is combustion with oxygen. The first 10 normal alkanes: Name Molecular formula drawing (stick) 1 methane CH4 2 ethane 3 propane 4 butane 2 5 pentane 3 6 hexane 5 7 heptane 9 8 octane 18 9 nonane 35 10 decane 75 # isomers Page 3 Organic Chemistry Structural formulas can be written as two- dimensional Lewis Structure formula, condensed structural formula, and as three- dimensional Valence Shell Electron Pair Repulsion (VSEPR) structure using a cabon skeleton formula, ball and stick model, or space filling model. In addition to normal alkanes, isomers are possible for all alkanes with 4 or more carbons. Example 1: Draw and name 9 possible structural isomers of C7H16 as Lewis structures. Alkanes can branch off. a a a a carbon is attached to one other carbon. carbon is attached to two other carbons. carbon is attached to three other carbons. carbon is attached to four other carbons. Page 4 Organic Chemistry CYCLOALKANES are alkanes that join the carbon chain together in a ring structure, (CnH2n). The smallest cycloalkane has 3 carbons and is called cyclopropane This structure may be drawn as a triangle in a condensed form where each point represents a carbon with the appropriate number of hydrogen atoms. Cycloalkanes with less than 5 carbons in a ring are strained since the angles are less than the ideal 109.5°. The most common and stable one is cyclohexane, C6H12, which can flex into chair and boat configurations that keep angles at 109.5° The most important chemical property of alkanes is combustion in oxygen to produce heat, carbon dioxide, and water. Apart from the above reaction alkanes are chemically inert. ALKENES (also known as olefins) The general formula for an alkene is CnH2n These unsaturated compounds contain a C=C double bond. The simplest alkene, called ethene or more commonly ethylene, contains 2 carbons, (C2H4). Ethylene is a plant hormone and is important in the ripening process of fruit. Alkenes may have cis and trans isomers. Example 2: Draw the cis and trans isomers of 2-butene… cis-2-butene trans-2-butene Example 3: Name the two structures below. Page 5 Organic Chemistry Alkenes undergo Addition Reactions. Markovnikov's rule applies to reactions when there are two possible products. This rule states that “the rich get richer”… carbon with more H preferably take an additional H. Example 4: Finish the reactions indicating the preferred product a) addition with H2 CH2=CH2 + H2 __Pt, Pd or Ni 500°C b) addition with Cl2 or Br2 (the Br2 addition tests for unsaturation) CH2=CH2 + Br2 c) addition with HX where X is a halogen preferred What is the other possible product, explain why it is less likely? d) addition with H2O CH2=CH-CH3 + H2O _H2SO4 ALKYNES The general formula for an alkyne is CnH2n-2 These unsaturated hydrocarbons have a C≡C triple bond. The simplest alkyne contains 2 carbons and is called ethyne or more commonly acetylene. C2H2. Alkynes also undergo addition reactions though less readily. Since the product still has a double bond, it is often possible to get a second addition reaction. Example 5: Draw the addition reaction of C2H2 with HCl. Page 6 Organic Chemistry AROMATIC HYDROCARBONS In an aromatic hydrocarbon, 6 carbon atoms are connected in a planar ring structure, joined by 6 sigma and 3 pi bonds (resonance) between carbon atoms. Benzene (C6H6) is the simplest aromatic hydrocarbon. Aromatic hydrocarbons undergo Substitution Reactions. When two groups are attached on a benzene ring three isomers are possible. ortho meta para Example 6: a) Draw and name the three isomers of the benzene ring in which two hydrogen atoms have been replaced by chlorine atoms. b) Draw the aromatic substitution reaction whose reactants are benzene (C6H6) and nitric acid (HNO3) the catalyst is sulfuric acid (H2SO4) and the products are nitrobenzene (C6H5NO2) and water (H2O) c) Draw the substitution reactions on benzene with Br2. (halogenation ) Page 7 Organic Chemistry FUNCTIONAL GROUPS: • Other organic compounds are hydrocarbons in which functional groups have been substituted for hydrogens • A functional group is a group of atoms that show a characteristic influence on the properties of the molecule, generally, the reactions that a compound will perform are determined by what functional groups it has. • Because the kind of hydrocarbon chain is irrelevant to the reactions, it may be indicated by the general symbol R. ALCOHOLS The general formula for an alcohol is R-OH R represents any alkyl group (CxHy). The simplest alcohol contains 1 carbon and is called methanol Alcohols are neutral compounds. Small alcohols are soluble in water. They have relatively high boiling points when compared to alkanes or ethers. Ethanol is an important alcohol used in drinks and as an antiseptic. PHENOLS For a phenol, R-OH ,the R is an aryl group (based on a benzene ring, C6H5OH). The simplest phenol is phenol (C6H5OH) where the group name comes from. ETHER The general formula for an ether is R-O-R The R and R' may be the same or different. The simplest ether is dimethyl ether For complicated ethers with more than one functional group we use the general name alkoxy, where alkoxy may be methoxy if there is 1 carbon, ethoxy if it has two carbons, etc. Ethers, like alkanes are virtually inert. This property makes them valuable as solvents. Two important chemical properties of ethers that laboratory workers must be aware of are 1) highly volatile and 2) highly flammable THIOLS, THIOETHERS The general formula for a thiol (mercapton) is R–S–H. The general formula for a thioether (sulfide) is R – S– R . In these compounds the oxygen in alcohols and ethers is replaced with a sulfur. Thiols smell like rotten eggs. Page 8 Organic Chemistry ALDEHYDES and KETONES The general formula for an aldehyde is R – CO–H. The simplest aldehyde is called formaldehyde The general formula for an ketone is R – CO–R. The simplest ketone is called Propanone which is commonly called acetone. Aldehydes may be prepared by the oxidation of primary alcohols Ketones may be prepared by the oxidation of secondary alcohols. Reduction (addition of H2) of aldehydes and ketones make primary and secondary alcohols in that order. Aldehydes are easily oxidized even under mild conditions. Ketones resist oxidation. This fact allows simple tests to tell these compounds apart. Aldehyde odors and flavors: Ketone odors and flavors: butanal = butter acetophenone = pistachio vanillin = vanilla carvone = spearmint benzaldehyde = almonds ionone = raspberries cinnamaldehyde=cinnamon muscone = musk Page 9 Organic Chemistry Carbonyl group C=O The carbonyl group may undergo addition reactions. Unlike the C=C double bond the C=O double bond is polar. Indicate the positive and negative ends of C=O and H-CN. CARBOXYLIC ACIDS and ESTERS Carboxylic acids are sour tasting weak acids. The general formula for a carboxylic acid is R – COOH. The simplest carboxylic acid is called methanoic acid or formic acid Esters have a sweet odor. The general formula for a carboxylic ester is R – COOR. The simplest carboxylic ester is called methyl formate It is useful to think of a carboxylic ester as being derived from a carboxylic acid part R-CO(OH) and an alcohol part (H)-O-R', as in the synthesis of aspirin… In naming carboxylic esters, do not try to name the ester as a whole. Name the root carboxylic acid and change the suffix "ic acid" to "ate". For example acetic acid becomes acetate. The first word is the name of the R' group in alcohol part. Example 7: Name CH3-C-O-CH2CH3 || O Page 10 Organic Chemistry AMINES Amines smell bad. They form when proteins decompose. They are organic bases. Many amines are biologically active (dopamine, epinephrine) The general formula for an amine is R-NH2 The simplest amine is called methylamine AMIDES The general formula for an amide is R-CO-NH2 The simplest amide is called formamide POLYMERS: Polymers are very large molecules (macromolecules) made by repeated linking together of small molecules (monomers) Natural polymers are found in both the living and nonliving environment. Synthetic polymers are polymers made in a lab from one, two, or three small molecules linked in a repeating pattern (plastics, fabrics, adhesives) Composites are materials made of polymers mixed with various additives such as graphite, glass, metallic flakes Polymerization is the process of linking the monomer units together addition polymerization of vinyl chloride (CHCl=CH2) PVC= polyvinylchloride condensation polymerization Page 11 Organic Chemistry Example 8: Identify the functional groups in caffeine. Chirality in Organic Chemistry A molecule possessing a nonsuperimposeable mirror image is CHIRAL. These compounds must have 4 different attached groups to a carbon (chiral center). R (right-handed) and S (left handed) labels distinguish the two forms we call enantiomers. Enantiomers have identical physical properties and identical chemical behaviors in nonchiral environments. Racemic mixtures have equal amounts of both R and S enantiomers in a mixture. Many drugs have only one effective enantiomer and the other is inert, an example is R-Albuterol useful as a bronchodilator for asthmatics, the L Albuterol is not effective. Biochemistry Biochemistry is the chemistry of living organisms. Amino Acids, the building blocks of proteins, have an amine and a carboxylic acid. 22 amino acids are found in nature and 20 are involved in making the proteins in our body. Page 12 Organic Chemistry Organic Nomenclature Rules: Saturated hydrocarbons - Alkanes: (CnH2n+2) 1) Find the longest continuous carbon chain and assign the parent name (1=methane, 2=ethane, 3=propane, 4=butane, 5=pentane, 6=hexane, 7=heptane, 8=octane, 9= nonane, 10= decane, 12=dodecane) i.e. if the longest chain is 5 carbons the end of the word will be pentane 2) Find groups not part of a chain and name as prefixed i.e. CH3 is methyl 3) Assign numbers to the carbons in the longest chain so attached groups have the lowest possible numbers i.e. if the choice is 2 or 4 choose 2 4) If 2 or more identical groups are on one compound use prefixes but remember to give a carbon chain number for each group; 2=di, 3=tri, 4=tetra, 5= penta, 6= hexa etc. 5) If 2 or more different groups are in the compound alphabetize i.e. chloro before fluoro and for carbon groups: ethyl before methyl 6) Halogens go by F = fluoro, Cl = chloro, Br = bromo, I = iodo 7) For cycloalkanes, (CnH2n) begin counting carbons at any position and proceed in either direction but you must end up with the lowest possible numbers. If only one substituent is connected in a cycloalkane there is no need to number the carbon 1 8) Also, for cycloalkanes there is a possible for stereoisomers since the ring structure will not permit free rotation. Stereoisomers may be labeled cis- for 2 groups on the same side and trans- for 2 groups on the opposite side. Unsaturated hydrocarbons - alkenes (CnH2n) and alkynes (CnH2n-2): 1) The suffix ene is used to represent a double bond between carbons the simplest is H2C=CH2 called ethene or more commonly ethylene (use the same prefixes as in the alkanes just change ane to ene. 2) The suffix yne is used to represent a triple bond between carbons the simplest is HC=CH called ethyne or more commonly acetylene 3) When the parent chain has more than 3 carbons a number position must identify where the double or triple bond is located using the lowest possible number i.e. if a double bond in a 5 carbon chain is located between the 2nd and 3rd carbon it is called 2-pentene 4) Branches and halogen substituents are names as in rules for alkanes 5) the longest continuous carbon chain must include the double or triple bonded carbons even though it may not be the longest chain. i.e. try to draw the structure for 2-methyl-1-butene 6) for cyclic compounds the double or triple bond will always be between carbon 1 and carbon 2. try drawing 4-bromo-2,3-dimethylcyclohexene 7) Compounds with 2 or more double bonds have prefixed suffixes; i.e. 2 double bonds will be diene, 3 = triene. Try to draw 2-methyl-4-ethyl-1,3-heptadiene. 8) Since the C=C double bond cannot freely rotate one may get stereoisomers (this will not happen in triple bonds) indicate cis- (H on same side) or trans- (H on opposite side) just before the number position of the double bond if it is a geometric isomer. Try to draw cis-2 hexene and trans-2-hexene. Aromatic hydrocarbons - not unsaturated but has double bonds (includes a benzene ring) 1) The simplest is benzene (C6H6) which is a six carbon ring where every other C-C bond may be written as a double bond in resonance. In reality the pi bonding is equally shared among Page 13 Organic Chemistry 2) 3) 4) all carbons which makes a very stable compound not willing to undergo addition reaction. Aromatic carbons undergo substitution reactions. If only 1 group is attached no carbon numbers are needed if 2 groups are attached the following rules apply a) if the groups are on neighboring carbons o- is used for ortho-, or may use the numbers1,2b) m- is used for meta-, or use the numbers 1,3c) p- is used for para-, or use the numbers 1,4If more than 2 groups just use the numbers that give the lowest sum. Alcohols and Phenols: (R-OH) For common names give the name of the alkyl group followed by the word alcohol i.e. methyl alcohol = CH3OH For IUPAC naming use the following rules: 1) The longest chain must include the carbon bearing the OH group 2) the suffix ol represents the OH group and the lowest possible number is assigned to this carbon. Try to draw 2-butanol 3) Alcohols may be classified as primary, secondary, or tertiary alcohols depending on how many other C atoms are attached to the same carbon that the OH group is attached to. This classification is important in reactions. 4) Phenols are aromatic compounds (benzene ring) which have a OH group 5) In complex compounds that have other suffixes hydroxy is used to indicate the OH group Ethers: 1) 2) 3) 4) (R-O-R') Give names of the R (organic) groups and add the word ether, i.e. ethyl methyl ether If groups are the same use di ether, i.e. diethyl ether (some people omit the di) For more complicated ethers use the alkyl group name followed by oxy then the other group. Try to draw 2-methoxycyclopentanol Cyclic ethers do not have ether in there names and follow rule 3. Thiols (R-SH) and Thioethers (R-S-R) 1) These are analogous to the alcohols and ethers but S replaces the O. Naming is analogous but use the term thiol not ol or alcohol and thioether not ether (C=O is a carbonyl group that is found in aldehydes, ketones, carboxylic acids, carboxylic esters, and amides) Aldehydes (R-CH=O) and Ketones (R-COR') 1) Same rules as apply for others above 2) suffix = al for aldehydes, Carbon number is ALWAYS 1 for the aldehyde carbon. if an alcohol of ether is also part of the compound then use hydroxy for the OH group and oxy for the ether. 3) suffix = one for ketones Page 14 Organic Chemistry 4) If there is both a alkene or alkyne and a aldehyde or ketone both suffixes may be used. i.e. try to draw 1-chloro-4-penten-2-one where a chlorine atom is attached to the 1st carbon the double bond is between carbons 4 and 5 and the carbonyl (C=O) group is on the 2nd carbon. Carboxylic acids (R-COOH): 1) Suffix oic acid other rules above are the same 2) the COOH carbon is always carbon number 1 3) The above IUPAC systematic naming is commonly used only for complicated compounds normally the common names are used examples: H-COOH Formic acid (Latin word for ant (formica) since 1st isolated from red ants) CH3COOH acetic acid CH3CH2COOH propionic acid CH3CH2CH2COOH butyric acid CH3(CH2)3COOH valeric acid CH3(CH2)16COOH stearic acid HOOC-COOH oxalic acid C6H5-COOH benzoic acid Carboxylic Esters (RCOOR'): 1) The first word comes from the Alcohol part of the word (The R' which is connected to by a single bond to an oxygen.) Name it as the alkyl group i.e. methyl 2) The second word is from the carboxylic part of the word ( the R which has the C=O carbonyl group) the "ic acid" in the common form becomes ate. acetic acid ==> acetate. Try to draw the structure methyl acetate. Amines (R-NH2, R-NHR', or NR3): 1) Add the suffix amine to the alkyl group i.e. CH3NH2 = methylamine 2) The simplest aromatic one is aniline C6H5NH2 3) For secondary and tertiary amines place all the R group names before the suffix amine and use di and tri as necessary. 4) When 1 R group is aromatic the amine is replaced with aniline 5) In complex compounds with competing suffixes the NH2 group is called "amino". Amides (R-CO-NH2, R-CO-NHR', R-CO-NR'2): (CO is a C=O) made by the dehydration of an amine and carboxylic acid Amides are named after there corresponding carboxylic acids; "ic acid" amide, i.e. CH3CO-NH2 is acetamide Page 15 Organic Chemistry Practice Problems: 1. A primary carbon is bonded directly to: (a) 1 hydrogen (b) 1 carbon 2. (c) 2 carbons (d) 3 carbons (e) 2 hydrogens What functional groups are present in the following? CH3-C-CH2-CH-CH=CH-CH3 ? ll l O OH (a) (b) (c) (d) (e) ketone, alkene, alcohol carboxylic ester, alkene, alcohol aldehyde, alcohol, amine phenol, carboxylic acid, alkene amide, ether, thiol 3. What is the geometry of propane? a) Planar with bond angles of 120°. b) Three-dimensional with bond angles of 90°. c) Three-dimensional with bond angles of 109.5°. d) Three-dimensional with bond angles of 120°. e) Three-dimensional with bond angles of 180°. 4. Why is the following name incorrect? 4-ethyl-5-methylcyclohexane a) b) c) d) 5. It is not named with the lowest possible numbers. Cycloalkanes do not contain alkyl groups attached to the ring. Ethyl and methyl groups are not found adjacent to one another on a ring. There is no such compound How many hydrogen atoms are there in 1-chloro-2 methylbenzene a) 3 b) 4 c) 5 d) 6 e) 7 f) 8 g) 9 6. Alkenes may have cis and trans isomers. Draw and identify the cis and trans isomers of 3-heptene 7. How can one test for saturation or unsaturation of hydrocarbons, include observations one would see? 8. When is Markovnikov's rule needed and what does is say? 9. Draw and name all possible structural isomers with the formula C6H14 Page 16 Organic Chemistry 10. Reactions: Finish and balance the following reactions, classify type of reaction as Addition, Substitution, or Combustion, and if 2 possible products are formed indicate the preferred product. + HNO H+ 3 a) Type b) CH2=CH2 + Br2 > Type c) C3H8 + O2 ____> Type d) CH3_CH=CH2 + H2O __H2SO4__> Type 11. Draw the Structures: If you skip the Hydrogens at least put a line to indicate where H belongs and how many. (a) (b) (c) (d) 12. m-dichlorobenzene or 1,3 - dichlorobenzene 2,2,3,3-tetrabromo-4-methyl-1-octanol methyl acetate 3-ethyl-3,4,6-trimethyl-2-decanone Name the Structures: (a) Cl l CH | CH3 (b) CH 3 -O-CH 2 -CH 3 (c) O-H | C l Cl CH3 | C CH- C H 2 -C H 3 l l CH3 C H2 - C H 2 - C H 3 H-C-CH2-CH-CH2-CH3 ll l O CH3 Page 17 Organic Chemistry (d) CH3-C-NH2 ll O 8. Circle and identify all the functional groups in the structural formula for the low-calorie sweetener aspartame below. List the functional groups here. For easier identification, number each circled group 9. Name the Structures: a) b) 10. CH3-O-CH3 O || C H 3 - C - O -C H 3 Draw and name all of the structural isomers with the formula C6H14 . Reactions: Finish the following reactions and classify type of reaction as Addition, Substitution or Combustion. 11. a) + HNO 3 H+ Type b) CH3- CH2-C-H ll O 12. + H2 __Ni__> Type Draw the Structures: (a) 2-chloro-3-hydroxy-4-methyl octanal (b) ortho-dichlorobenze (c) 3-ethyl-2- hexanone Page 18








