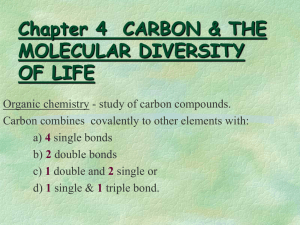Topics
advertisement

Basic Chemistry (elective course) Topics of credit test 1. s- and p-elements - group name, metal / nonmetal, number of valence electrons, common oxidative states 2. symbols and names of common elements (both English and Latin) 3. oxidative state of an element in a compound 4. names of common inorganic cations nad anions 5. distinguish: element / molecule / cation / anion (using their names) 6. naming of oxides, peroxides, hydroxides, acids and salts 7. balance a chemical equation (not redox) 8. writte an equilibrium constant of a reaction (an equation) 9. add products to given inorganic reaction (neutralization, oxidation, reduction, dissociation of an acid) or give type of the reaction 10. electronegativity, type of bonds 11. organic compounds: structural formulas of hydrocarbons (saturated, unsaturated, aromatic) and their derivatives (i.e. structure of functional groups) 12. choose polar / nonpolar / amphipatic compounds (from their names) 13. choose saturated / unsaturated compounds (from their names) 14. isomers (draw formulas – organic compounds) 15. systematic names of organic acids and their anions and acyls 16. systematic names of aldehydes, ketones, alcohols, amines, ethers, halogen and nitroderivatives 17. add products to given organic reaction (addition, elimination, hydrogenation, dehydrogenation, dissociation of an acid) calculation without a calculator: 18. calculation based on chemical reaction (e.g. masses of reacting compounds) 19. calculate relative molecular mass 20. calculate molar or % concentration from given masses 21. calculate mass of a substance needed for preparation of a solution of given concentration (molar or %) 22. calculate osmolarity / molarity of a solution 23. calculate concentration of H3O+ and OH- in an aqueous solution 24. calculate pH of a strong acid or base 25. calculate pK from Ka or vice versa (or pH / cH+) The test contains 25 questions, you will create the answer (it is not a multiple choice test). You need 60% of points at least.