Percent Composition Notes
advertisement

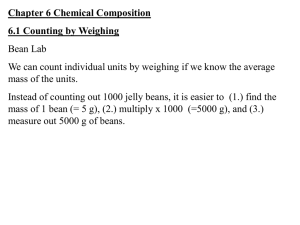
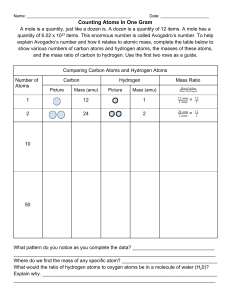
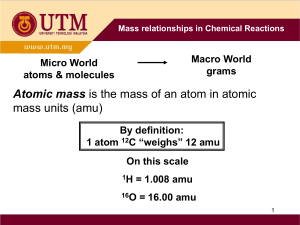
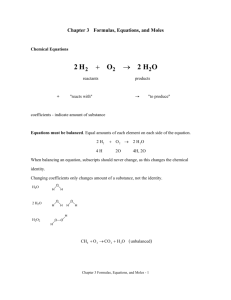
Percent Composition – Notes Key Vocabulary Formula mass – the sum of the average atomic masses of all atoms represented in a molecule’s formula Percentage composition – the percentage by mass of each element in a compound Input Formula Masses (amu) To find the formula mass find the amu (atomic mass unit) of each element in the formula. Next multiply the amu for each element by the number of each element in the formula. Finally add them all together. Examples: H20 has the elements hydrogen (amu = 1.01) and oxygen (amu = 16.00). (2 x 1.01) = 2.02 for hydrogen then add 16.00 for oxygen. Finally the average mass for H20 is 18.02 amu Molar Masses (g/mol) Molar mass is calculated exactly the same as formula mass. The difference is that molar mass units are grams per mole while formula mass units are amu’s. Conversions Grams use molar mass moles Moles use Avogadro’s number formula units Examples: How many copper atoms in a penny that weighs 3g? Grams use molar mass moles 3g Cu(1 mol/63.5 g Cu) = .047244 moles of Cu Moles use Avogadro’s number formula units .047244 moles of Cu (6.022 x 1023 atoms) = 2.84 x 1022 Cu atoms How many glucose molecules (C6H12O6) are in 5.23 g of glucose? Answer = 1.75 x 1022 molecules C6H12O6 How many oxygen atoms are in the glucose sample? Answer = 1.05 x 1023 atoms O Percentage Composition To find the percentage of mass of an element in a compound First find the molar mass of the compound Second divide the mass of the element by the mass of the compound Third multiply by 100 to get the percentage Examples: Find the percentage of chlorine in lead (II) chloride (PbCl 2) Pb = 207.2 g Cl = 35.5 g times 2 atoms = 71 g PbCl2 = 278.2g 71g divided by 278g = .2554 Multiply by 100 = 25.5% chlorine Find the mass percentage of water in the hydrate CuSO4*5H2O Answer = 36.08% Find the percentage composition of the following Ba(NO3)2 Answer = 52.55% Ba, 10.72% N, 36.73% O Guided Practice: Sample problems p 238 (Finding formula mass) Sample problems p 239 (Finding molar mass) Sample problems p 240 - 242 (Grams) Sample problems p 243 - 244 (Percent composition) Resources Modern Chemistry text (Holt, Rinehart, and Winston) Page 237 – 244